


Elevating Allergen Testing: Capabilities, Challenges, and the Path Forward
Elevating Allergen Testing: Capabilities, Challenges, and the Path Forward Presented by Jayaraj Alappat, John Servello and Tim Lombardo August 19, 2025, 12:00 pm (eastern) Food allergies are a growing public health concern, affecting millions and shaping the way food...cGMP Responsibilities for Own Label Distributors and Brand Owners
cGMP Responsibilities for Own Label Distributors and Brand Owners Presented by Shelly Blackwell December 18, 2025, 1pm (eastern) Own Label Distributors (OLD) in the dietary supplement industry often question their responsibilities for complying with current Good...
FDA Encourages Food Manufacturers to Accelerate Phasing Out the Use of FD&C Red No. 3 in Foods Before 2027 Deadline
July 14, 2025 View on FDA Website On January 15, 2025, the U.S. Food and Drug Administration issued an orderrevoking the authorization for the use of FD&C Red No. 3 in foods, including dietary supplements (permitted under 21 Code of Federal Regulations (CFR)...
Overview of Regulatory Requirements for OTC Monograph Drugs
Overview of Regulatory Requirements for OTC Monograph Drugs Presented by Victoria Pankovich September 17, 2025, 1pm (eastern) If you are considering entering the OTC monograph drug space, the challenges of FDA regulatory requirements can be confusing. With a mixture...
Fernanda Estefanía Jiménez Garzón
Food Engineer with specialized experience in food safety, thermal processing, and quality assurance systems. Fernanda has worked on the design and validation of thermal processes for low acid and acidified foods, in compliance with FDA regulations (21 CFR 113 and...
María Fernanda Mandujano
Highly motivated and committed Food Engineer with a strong background in food safety systems, international regulatory compliance (FDA, FSMA, USDA), product development, and operational leadership. She brings a hands-on approach to problem-solving, ensuring regulatory...
Carolina Herrera Samaniego
Carolina Herrera Samaniego is a regulatory compliance consultant specializing in food, dietary supplements, cosmetics, and OTC products for international markets. She holds a degree in Nutrition and Food Science from IBERO Puebla, and has developed expertise in FDA...
Tanguy Etoga
Tanguy Etoga, holds a Bachelor’s degree in Food Science and Technology from Laval University and a Master’s degree in Operations Management from McGill University in Canada. He has over 20 years of experience in the food industry supporting businesses in achieving and...
US Food and Drug Administration FDA Embraces Radical Transparency by Publishing Complete Response Letters
FDA News Release July 10, 2025 View on FDA Website The U.S. Food and Drug Administration (FDA) today published more than 200 decision letters, known as complete response letters (CRLs). The CRLs were issued in response to applications submitted to the FDA for approval...
Comparative Perspective on Thermal Processing: Regulations and Practices in Mexico and the U.S.
Comparative Perspective on Thermal Processing: Regulations and Practices in Mexico and the U.S. By Fernanda Estefanía Jiménez Garzón, EAS Consulting Group Independent Consultant What if the foodborne illness you experienced… could have been prevented? According to the...
Conducting Remote Regulatory Assessments Questions and Answers
Download the Final Guidance Document Read the Federal Register Notice Docket Number: FDA-2022-D-0810 Issued by: Office of Inspections and Investigations Center for Biologics Evaluation and Research Center for Devices and Radiological Health Center for Drug Evaluation...
Foods Program FDA Guidance Under Development
The following list of guidance topics includes possible new topics for guidance documents or revisions to existing guidance documents that the FDA is considering for Human and Animal Foods. The lists below include some of the guidance documents under consideration to...
FDA Updates General Food Labeling Requirements Compliance Program
Constituent Updates June 24, 2025 Read on FDA Website The U.S. Food and Drug Administration has updated Compliance Program 7321.005, now titled General Food Labeling Requirements and Labeling-Related Sample Analysis – Domestic and Import. This update replaces the...
Drug and Device Corner June 2025
The FDA has released two proposed administrative orders for requesting minor dosage form changes to certain OTC monograph drugs. These administrative orders are open for comment. Proposed Administrative Order OTC000038 If finalized, this proposed order will specify...
FDA Seeks Input on a New Method for Ranking Chemicals in Food for Post-market Assessments
Constituent Update June 18, 2025 Today, the U.S. Food and Drug Administration (FDA) released for public comment its proposed method for ranking chemicals in the food supply. This method provides a transparent, systematic, and science-based approach to determine which...
FDA to Issue New Commissioner’s National Priority Vouchers to Companies Supporting U.S. National Interests
For Immediate Release: June 17, 2025 The U.S. Food and Drug Administration today announced its Commissioner’s National Priority Voucher (CNPV) program to enhance the health interests of Americans. The new voucher may be redeemed by drug developers to participate in a...
Raising Your Nutrition Label IQ
Many people have difficulty understanding the Nutrition Facts label, especially with regards to properly interpreting and using the information about serving sizes and the percent Daily Values. This webinar will clear up some of the confusion about these and other labeling issues. This webinar will also discuss some of the science that went into developing the Nutrition Facts label and explain why FDA decided to update and modernize the label in 2016. By attending this webinar, you will become a more knowledgeable label reader.

Navigating Regulatory Shifts: The Importance of Quality Management in the Tobacco Industry
Navigating Regulatory Shifts: The Importance of Quality Management in the Tobacco Industry by Gabriel Muñiz, EAS Consulting Group Independent Consultant The tobacco industry is navigating a landscape of evolving regulations, prompting companies to reconsider their...
A Guide to FSMA 204
A Guide to FSMA 204: Sorting Through the Confusion of the FDA’s New Traceability Rule for Added Food Safety Presented by Tim Lombardo and Thomas Bell September 24, 2025, 1pm (eastern) The final Food Safety Modernization Act rule, the Food Traceability Rule – commonly...
Drug and Device Corner May 2025
Reminder that OMUFA FY2025 facility user fees are due Monday 2 June 2025. MDF fee is $37,556; CMO fee is $25,037. Please see the FDA website for full details. Do note below the agency will be providing an OMUFA user fee and registration webinar Tuesday 20 May 2025....
Dietary Component Specifications and Testing
Since the FDA’s dietary supplement cGMP regulations (21 CFR Part 111) were published, specifications and testing have been consistently one of the most frequently cited FDA observations. Specifically, industry continues to face challenges in complying with the specification and testing requirements for dietary supplement components.

HHS, FDA Issue RFI on Deregulatory Plan to Lower Costs and Empower Providers
HHS, FDA Issue RFI on Deregulatory Plan to Lower Costs and Empower Providers May 13, 2025 View on the FDA Website The U.S. Department of Health and Human Services (HHS) and the U.S. Food and Drug Administration (FDA) announced the launch of a public Request for...
FDA Approves Three Food Colors from Natural Sources
May 09, 2025 The U.S. Food and Drug Administration announced it granted three new color additive petitions that will expand the palette of available colors from natural sources for manufacturers to safely use in food. The FDA is in line with U.S. Department of Health...
Front-of-Package Nutrition Information; Extension of Comment Period
In response to requests for an extension, the FDA is extending the comment period for the proposed rule entitled ‘‘Food Labeling: Front-of-Package Nutrition Information’’ that appeared in the Federal Register of January 16, 2025, by 60 days. Either electronic or...
Animal Feed Regulatory Pathways
Animal Feed Regulatory Pathways by Adam Orr, EAS Consulting Group Independent Consultant As you know, food is regulated by the FDA under the Federal Food Drug and Cosmetic Act (FDCA). In brief, it defines food as articles used for food or drink for man or other...
FDA Announces Expanded Use of Unannounced Inspections at Foreign Manufacturing Facilities
May 06, 2025 Today, the U.S. Food and Drug Administration announced its intent to expand the use of unannounced inspections at foreign manufacturing facilities that produce foods, essential medicines, and other medical products intended for American consumers and...
Drug and Device Corner April 2025
Under GDUFA, human generic drug facilities, sites, and organizations are required to submit identification information electronically to FDA annually. These submissions are made during the month of May The following types of generic industry facilities, sites, and...
Special Alert: FSIS Withdraws Proposed Salmonella Framework for Raw Poultry Products
Special Alert: Constituent Update April 24, 2025 FSIS is withdrawing its proposed rule and determination titled “Salmonella Framework for Raw Poultry Products”, published on August 7, 2024, to further assess its approach for addressing Salmonella illnesses associated...
HHS, FDA to Phase Out Petroleum-Based Synthetic Dyes in Nation’s Food Supply
For Immediate ReleaseApril 22, 2025 The U.S. Department of Health and Human Services and U.S. Food and Drug Administration (FDA) today announced a series of new measures to phase out all petroleum-based synthetic dyes from the nation’s food supply—a significant...
Transforming Your Food Safety and Regulatory Compliance Programs with Artificial Intelligence
Simply put, Artificial Intelligence (AI) is the ability of a computer to perform tasks commonly associated with humans. In the area of food safety and food regulatory compliance, there are many tasks in which AI applications can be used, some more advanced than others.

In Memoriam: Bruce Elsner
In Memoriam: Bruce Elsner We have sad news to share on the passing of Bruce Elsner who was a long-time member of the EAS family and consulting team. Bruce had over 28 years of experience working in FDA regulated Fortune 500 companies, who manufactured and packaged...
FDA hiring contractors to replace fired staff who supported safety inspections
The FDA is planing to hire contractors to replace fired staff who supported safety inspections, including those who arranged foreign travel and tested food samples. The cuts, which include positions in communications and policy, are expected to strain the...
EAS Consulting Group Expands Expertise with the Addition of Two Former FDA Staffers
EAS Consulting Group is pleased to announce the addition of two highly accomplished former FDA staff members, Gabriel Muñiz and Adam Orr, to its team of independent consultants. Their expertise in regulatory compliance and experience in the FDA’s tobacco and animal feed sectors will further strengthen EAS’s ability to provide superior guidance to clients navigating complex regulatory landscapes.

Drug and Device Constituent Update 2025 April
We want to keep our clients up to date as we learn of any changes at the FDA that will affect normal business processes. The following 3 points have come to our attention in the last few days. For any questions regarding the status of a product in the NDC Directory,...
Health Claims and Dietary Fiber
Health Claims and Dietary Fiber by Mark Kantor, EAS Consulting Group Independent Consultant Health claims are allowed voluntarily in food labeling if sufficient scientific evidence exists that a specific food or food component – referred to as a “substance” by FDA –...
Major Shakeup at FDA Continues as Tobacco Chief Removed
We wanted to share this important AP News article about the latest developments at the FDA, where tobacco director Brian King has been placed on administrative leave amid sweeping cuts to the federal health workforce. Key developments: King, who joined the FDA in...
HHS Restructuring and Organizational Streamlining
March 28, 2025View on the HHS Website The U.S. Department of Health and Human Services (HHS) announced significant organizational changes. Key Highlights of the HHS Restructuring: Organizational Transformation Workforce reduced from 82,000 to 62,000 employees...
Webinar on the Updated “Healthy” Claim
March 27, 2025View on the FDA Website The FDA Webinar on the Updated “Healthy” Claim has been rescheduled for April 10, 2025, from 1-2 pm EDT. Please note, the webinar is open to new registrants, but you do not need to re-register if you previously signed-up for this...
New Searchable Web Page for the Food Allergen Labeling Guidance for Industry
March 26, 2025 View on the FDA Website Today, the FDA published a web page, Frequently Asked Questions: Food Allergen Labeling Guidance for Industry, as a resource for the 5th edition of FDA’s Guidance for Industry titled: Questions and Answers Regarding Food...
HHS, FDA Announce Operation Stork Speed to Expand Options for Safe, Reliable, and Nutritious Infant Formula for American Families
For Immediate Release: March 18, 2025View on FDA Website Today, under the leadership of U.S. Department of Health and Human Services Secretary Robert F. Kennedy, Jr., the U.S. Food and Drug Administration is taking steps to enhance its efforts to ensure the ongoing...
Adam Orr
Adam Orr is a Certified Professional Animal Scientist and an Animal Nutritionist with a lifetime of experience in and around multi-species animal agriculture. The past 15 years have been spent at the FDA Center for Veterinary Medicine and the Division of Animal Food...EAS Consulting Group Welcomes Adam Orr as Independent Consultant
Adam Orr is a Certified Professional Animal Scientist and an Animal Nutritionist with a lifetime of experience in and around multi-species animal agriculture. The past 15 years have been spent at the FDA Center for Veterinary Medicine and the Division of Animal Food...
Drug and Device Corner March 2025
The 2025 OMUFA facility user fees have been announced in the Federal Register (see link below). Facility User Fee Rates FY 2025 Monograph Drug Facility (MDF) Fee $37,556 Contract Manufacturing Organization (CMO) Fee $25,037 The background and legislation on this...
Pet Supplements Unleashed: Navigating the Regulatory Maze
Pet Supplements Unleashed: Navigating the Regulatory Maze Presented by Kevin Ragland, EAS Consulting Group Independent Consultant Pet supplements and nutraceuticals are extensively used by dog, cat, and horse owners throughout the United States, contributing billions...
FDA Intends to Extend Compliance Date for Food Traceability Rule
Constituent Update March 20, 2025 View on the FDA Website Today, the U.S. Food and Drug Administration (FDA) is announcing its intention to extend the compliance date for the Food Traceability Rule (the “final rule”) by 30 months. The FDA intends to extend the...
HHS, FDA Announce Chemical Contaminants Transparency Tool for Foods
For Immediate Release: March 20, 2025View on FDA Website Today, under the leadership of U.S. Department of Health and Human Services Secretary Robert F. Kennedy, Jr., the U.S. Food and Drug Administration unveiled the Chemical Contaminants Transparency Tool (CCT...
Gabriel Muniz
Gabriel Muniz is a distinguished expert in FDA regulatory compliance, quality systems, and risk management, with nearly two decades of experience in tobacco and other FDA regulated product industries. Having served as a Director at the FDA and a senior regulatory...EAS Consulting Group Welcomes Gabriel Muniz as Independent Consultant
EAS is pleased to welcome Gabriel Muñiz to the EAS Consulting Group team of independent consultants. Gabriel is a distinguished expert in FDA regulatory compliance, quality systems, and risk management, with nearly two decades of experience in tobacco and other FDA...
Understanding the Role of Your U.S. Agent
Foreign entities who operate in US FDA-regulated industries must have an appointed FDA US agent before they can import products into the US. At a minimum these FDA US agents provide the most basic of required services. However, agents with relevant experience and knowledge can provide much more.

HHS Secretary Kennedy Directs FDA to Explore Rulemaking to Eliminate Pathway for Companies to Self-Affirm Food Ingredients Are Safe
The U.S. Department of Health and Human Services and the U.S. Food and Drug Administration are committed to promoting radical transparency to make sure all Americans know what is in their food. Today, as part of this commitment, HHS Secretary Robert F. Kennedy Jr. is...
Salmonella Framework for Raw Poultry Products
Salmonella Framework for Raw Poultry Products by Dionne S. Meehan, EAS Consulting Group Independent Consultant On August 7, 2024, the United States Department of Agriculture’s (USDA) Food Safety and Inspection Service (FSIS) published its updated “Salmonella Framework...
2024 CARES Act Drug Volume Reporting Reminder
Final reminder that CARES volume reports for 2024 are due no later than 31 March 2025 for all registered drug establishments. Do remember how vital it is to ensure all NDC listings reflect their correct status. The FDA will expect volume information for an NDC no...
Mark Kantor
Mark Kantor’s professional career has been at the intersection of food science and nutrition, including positions in government, academia, and industry. He was a nutrition scientist and regulation writer in FDA’s Office of Nutrition and Food Labeling for 14 years...
Drug and Device Corner February 2025
Reminder that CARES volume reports for 2024 are due no later than 31 March 2025. If you need assistance with the process, EAS is here to help. The FDA has updated the OTC MONOGRAPHS @ FDA website’s OTC Monograph Resources section. This includes a digital Catalogue of...
Food and Dietary Supplement Labeling: What Comes Next?
Labeling of food and dietary supplements in the United States involve several aspects and each must be approached with careful consideration. Regulatory, scientific, and business decisions need to be considered when working on labels’ mandatory elements and claims....
Food Standards Australia New Zealand
Food Standards Australia New Zealand (FSANZ) has compiled all 80 Standards and 29 Schedules of the Code into a single, easy-to-search and navigate guidance document. While an obvious resource for Australian and New Zealand based food businesses, it is also a great...
Cosmetic Registration and Listing Requirement Reminder
Now that we have entered the first year post cosmetic registration and listing requirements, it’s a good time to review your records and make sure you are prepared for your update / renewal process. For facility registrations, an update should be submitted within 60...
Dionne Meehan
Dionne Meehan served in the US Army for 16 years before retiring, then transitioned to private industry to become a recognized expert in FSIS, USDA, and FDA regulatory systems with a proven track record of securing FSIS regulatory exceptions, implementing innovative...
Audits: Internal, Regulatory, and Mock…. Oh, My!
Audits come in a variety of types, but the three main audit types that will be covered here are Regulatory Audits, Internal Audits, and Mock Audits. The ways a company handles, conducts and views these three categories of inspections will set them up for success. Read...
Regulatory Freeze Executive Order
Regulatory Freeze Executive Order Effective January 20, 2025, President Trump issued a Regulatory Freeze Executive Order, which will have a direct impact on regulated industries. The regulatory freeze order, consistent with the actions of previous incoming...
Drug and Device Corner January 2025
Effective January 20, 2025, The President issued a Regulatory Freeze Executive Order, which will have a direct impact on regulated industries. The regulatory freeze order, consistent with the actions of previous incoming administrations, effectively halts any...
Regulatory Freeze Executive Order
Effective January 20, 2025, President Trump issued a Regulatory Freeze Executive Order, which will have a direct impact on regulated industries. Regulatory Freeze The regulatory freeze order, consistent with the actions of previous incoming administrations,...
Cat and Dog Food Manufacturers Required to Consider H5N1 in Food Safety Plans
January 17, 2025Read on FDA Website The U.S. Food and Drug Administration has determined that it is necessary for manufacturers of cat and dog foods who are covered by the FDA Food Safety Modernization Act Preventive Controls for Animal Food (PCAF) rule and using...
FDA Issues Proposed Rule on Front-of-Package Nutrition Labeling
On January 14, 2025, the U.S. Food and Drug Administration (FDA) announced a proposed rule to require a front-of-package (FOP) nutrition label on most packaged foods to provide accessible, at-a-glance information to help consumers quickly and easily identify how foods...
A Year in Review: FDA’s Progress on Tobacco Product Regulation in 2024
With a new year upon us, FDA’s Center for Tobacco Products (CTP) continues to work diligently to protect the public health of the U.S. population from tobacco-related disease and death. At the same time, we also celebrate significant public health wins that have...
FDA Proposes Significant Step Toward Reducing Nicotine to Minimally or Nonaddictive Level in Cigarettes and Certain Other Combusted Tobacco Products
Today, the U.S. Food and Drug Administration issued a proposed rule that, if finalized, would make cigarettes and certain other combusted tobacco products minimally or nonaddictive by limiting the level of nicotine in those products. If finalized, the United States...
Guidance for Industry: Questions and Answers Regarding Food Allergen Labeling (Edition 5)
January 2025 Docket Number: FDA-2022-D-0099 Issued by: Human Foods Program Download the Final Guidance Document Read the Federal Register Notice Final Guidance for Industry: Questions and Answers Regarding Food Allergens, Including the Food Allergen Labeling...
Considerations for Complying with 21 CFR 211.110
January 2025 Docket Number: FDA-2024-D-5374 Issued by: Center for Drug Evaluation and Research Center for Biologics Evaluation and Research Center for Veterinary Medicine Download the Draft Guidance Document Read the Federal Register Notice This guidance, when...
Artificial Intelligence-Enabled Device Software Functions: Lifecycle Management and Marketing Submission Recommendations
Docket Number: FDA-2024-D-4488Issued by: Center for Devices and Radiological HealthCenter for Biologics Evaluation and ResearchCenter for Drug Evaluation and Research Download the Draft Guidance Document Read the Federal Register Notice This draft guidance document...
Pet Supplement Industry on the Rise
Recent survey reports indicate that supplements for pets have reached US sales of nearly $3 billion, with dog and cat supplements comprising of approximately 75% and 20% of the total, respectively. The growing pet supplement market is aimed at pet health and wellness, particularly for issues like mobility, skin and coat health, and anxiety. Survey reports specifically indicate that hip and joint supplements are the most frequently type of supplement purchased.

Guidance for Industry: Action Levels for Lead in Processed Food Intended for Babies and Young Children
January 2025 Docket Number:FDA-2022-D-0278 Issued by: Human Foods Program Download the Final Guidance Document Read the Federal Register Notice FDA is committed to reducing lead in food. FDA’s Closer to Zero initiative is a science-based, iterative approach to...
FDA Issues Final Guidance on Analytical Testing for Industry Use in Tobacco Product Applications
Agency remains committed to providing resources to support application submissions January 6, 2025 Today, FDA issued “Validation and Verification of Analytical Testing Methods Used for Tobacco Products,” a final guidance providing tobacco manufacturers with...
Claims Substantiation Seminar
This two-hour training will be a scientific dive into the regulatory requirement of claims substantiation. Several topics including the following will be discussed along with real case examples: which types of evidence are available and where to find them; what are high quality and low quality evidence; when is the evidence irrelevant to the claim being made; and how to write claims that can be substantiated.

Paula Brock, Ph.D., MCSI
Works directly with EAS’ Senior Director, Labeling, Cannabis and Claims Consulting Services to assure client satisfaction and project management. She collaborates with EAS consultants and clients to provide excellence in service. Dr. Brock facilitates food, dietary...
Drug and Device Corner December 2024
The FDA issued a final rule establishing requirements for a nonprescription drug product with an Additional Condition for Nonprescription Use (ACNU). This new marketing pathway will allow the sale of a nonprescription drug product directly to a consumer once the user...
FDA Proposes Rule to Require Standardized Testing Methods for Detecting and Identifying Asbestos in Talc-Containing Cosmetic Products
For Immediate Release: December 26, 2024 Today, the U.S. Food and Drug Administration (FDA) announced a proposed rule to establish and require standardized testing methods to detect and identify asbestos in talc-containing cosmetic products. This proposed rule, if...
FDA issues final rule to broaden types of nonprescription drugs available to consumers
Today, the U.S. Food and Drug Administration issued a final rule establishing requirements for a nonprescription drug product with an additional condition for nonprescription use (ACNU). A nonprescription drug product with an ACNU is a drug product that could be...
FDA Updates “Healthy” Claim, Providing a Refreshed Tool for Consumers
December 19, 2024 Today, the U.S. Food and Drug Administration is announcing a final rule to update the definition of the nutrient content claim “healthy.” There is an ever-growing crisis of preventable, diet-related chronic diseases in the U.S. that requires...
FSIS Announces Stronger Measures to Protect the Public from Listeria monocytogenes
WASHINGTON, December 17, 2024 – The U.S. Department of Agriculture’s (USDA) Food Safety and Inspection Service (FSIS) today announced several new steps to strengthen the agency’s oversight of food processing facilities it regulates (meat, poultry and egg products) and...
Infant Formula Regulations in Australia and the United States: A Brief Comparison
Infant Formula Regulations in Australia and the United States: A Brief Comparison By Julie Cottle – Regulatory Matters Global Technical & Compliance Specialist | Food & Complementary Medicines Infant formula is an important product for infants who cannot...
FDA Submits Proposal to Set a Nicotine Limit on Tobacco Products
The U.S. Food and Drug Administration has submitted a proposal to the Office of Management and Budget (OMB) to limit the amount of nicotine in tobacco products, reports CNN. “A proposed product standard to establish a maximum nicotine level to reduce the addictiveness...
FDA Updates Guidance for Industry on Registration and Listing of Cosmetic Product Facilities and Products
Read on FDA Site Cosmetics Constituent Update December 11, 2024 The U.S. Food and Drug Administration (FDA) issued updated guidance entitled Guidance for Industry: Registration and Listing of Cosmetic Product Facilities and Products. In summary, the guidance finalizes...
VQIP Application Portal Opens for FY2026
Constituent Update December 10, 2024 Read on FDA site On January 1, 2025, the U.S. Food and Drug Administration (FDA) will open the Voluntary Qualified Importer Program (VQIP) application portal for fiscal year (FY) 2026. VQIP is a voluntary fee-based program...
FDA Issues Guidance on Notifying FDA of a Permanent Discontinuance in the Manufacture or an Interruption of the Manufacture of an Infant Formula
Constituent Update December 2, 2024 The U.S. Food and Drug Administration (FDA) has issued draft guidance outlining the requirements and procedures that infant formula manufacturers should follow to notify the FDA of a permanent discontinuance or an interruption of...
FDA Issues 2024 Voluntary National Retail Food Regulatory Program Standards
Constituent Update December 2, 2024 The U.S. Food and Drug Administration (FDA) today issued the 2024 edition of the Voluntary National Retail Food Regulatory Program Standards, which defines the key elements of an effective retail food regulatory program for state,...
Drug and Device November 2024
This is the final reminder that we are in the annual medical device facility renewal, drug establishment renewal, and listing certification period. All renewals and certifications must be completed by 31 December 2024. If you need help with your submissions, please...
FDA Updates Guidance for the Voluntary Qualified Importer Program (VQIP)
Constituent Update November 14, 2024 The U.S. Food and Drug Administration (FDA) today released guidance with revisions to the FDA Food Safety Modernization Act (FSMA) Voluntary Qualified Importer Program (VQIP). VQIP offers importers who achieve and maintain a high...
Health Canada
Interested in doing business of health products in Canada? This free webinar provides an overview of the Canadian regulation on natural health products and how EAS can assist companies with the diverse requirements of Health Canada.

FDA Seeks Public Input on Experiences with Export Certification in the Form of Lists (Export Lists) for Human Food Products
Constituent Update November 7, 2024 The U.S. Food and Drug Administration (FDA) is seeking public input on its current approach to managing FDA certification for the export of human food products regulated by the agency. Firms exporting products from the U.S. are...
21 CFR Part 11 and FDA Compliance
This webinar will help you understand in detail Computer System Validation (CSV) and how to apply the System Development Life Cycle (SDLC) Methodology

Are You as an Importer Ready for FSMA Traceability Rule?
Are You as an Importer Ready for FSMA Traceability Rule? By Angel Suarez, EAS Consulting Group Independent Consultant Under the Food Safety Modernization Act (FSMA) the FDA has finalized several major rules to ensure the safety of the food supply is a shared...
Human Food Program (HFP) FY 2025 Priority Deliverables
On this page: Introduction FY 2025 Priority Deliverables About the Human Foods Program Introduction The FDA is responsible for regulating 80% of the U.S. food supply, with the Human Foods Program (HFP) overseeing all activities related to food safety and nutrition....
FDA Releases Supplement to the 2022 Food Code
The U.S. Food and Drug Administration (FDA) today has published the Supplement to the 2022 Food Code. The Supplement updates the 2022 Food Code with recommendations made by regulatory officials, industry, academia, and consumers at the 2023 Biennial Meeting of the...
Drug and Device October 2024
Reminder: We are now in the registration renewal period for both drug establishments and medical device facilities. Drug listing certifications must also be submitted between 1 October and 31 December annually. If you need assistance with the process, please contact...
Good ANDA Submission Practices
Good ANDA Submission Practices Proactive Planning for the Best Possible Outcomes Presented by Radhika Rajagopalan, Ph.D., EAS Independent Consultant FDA recent draft and final guidance documents related to generic drugs indicate the Agency’s thinking with regards to...
Food Traceability Rule (FSMA 204)
Food Traceability Rule (FSMA 204) Coming Soon! The FDA Food Safety Modernization Act (FSMA), enacted in 2011, empowers the FDA to bolster public health by strengthening the food safety system through new regulatory actions. FSMA is the compilation of many FDA food...
CVM GFI #294 – Animal Food Ingredient Consultation (AFIC)
The Federal Food, Drug, and Cosmetic Act (FD&C Act) gives FDA the authority to regulate substances used in animal food, including substances that are food additives and substances that are generally recognized as safe (GRAS) for their intended uses in food. Since...
Important Changes to OTC Monograph Listing Marketing Categories
In addition to a reminder of the Drug Establishment Registration Renewal period (1 October – 31 December) EAS would like to remind you of significant changes being implemented for OTC monograph drug product listings. The changes were announced by FDA last year and...
Investigating Pesticide Tolerance
Investigating Pesticide Tolerance By Dr. Bill Reeves, EAS Regulatory Consultant Consistent, scientifically justified regulations enable over $1.5 trillion in global agricultural exports every year. The world economy depends on these regulations to ensure whatever goes...
Bill Reeves
Dr. Bill Reeves is a toxicologist with experience in human health and environmental risk assessment. After earning his Ph.D. from Texas A&M University, Bill worked for California EPA establishing new water quality standards and conducting technical reviews of...
Drug and Device September 2024
The FDA Drug and Medical Device Registration renewal period begins 1 October 2024. With the drug listing certification period upon us, it is a good time to confirm your NDC listings are all accurate. Any NDC SPL file requiring updates should be done prior to 31...
FDA Reminds Animal and Human Food Facilities to Register or Renew their Food Facility Registration (FFR) between October 1 and December 31, 2024
Owners, operators, or agents in charge of a domestic or foreign facility engaged in manufacturing/processing, packing, or holding food for consumption by humans or animals in the U.S., are required to register the facility with the FDA. The registration and renewal...
Importing FDA-Regulated Seafood Products – New FDA Video Now Available
The U.S. Food and Drug Administration (FDA) has released the third video in the “Importing FDA-Regulated Products” series. This new video provides an overview of the process for importing seafood, focusing on key regulatory requirements. It is designed to help...
New Webinar: Preparing and Submitting a Standalone Pre-Existing Tobacco Product Submission
CTP invites you to watch the tobacco compliance webinar, “Pre-Existing Tobacco Product Determination Program Webinar Series Part 2 of 3: Preparing and Submitting a Standalone Pre-Existing Submission.” This webinar is part two of a three-part webinar series...
Claims Substantiation of Cosmetic Products
Claims Substantiation of Cosmetic Products An EAS Consulting Group Complimentary On Demand Webinar Presented by Paula Brock, Ph.D., MCSI Substantiation of product claims is required by law in the USA and this is not different for cosmetics. All types of claims need to...
FY2025 Drug Program User Fees
Please see below information regarding FY2025 Drug Program User Fees which go into effect 1 October 2024. The FDA has announced FY2025 user fees for GDUFA, OMUFA (Order Requests only), PDUFA, BsUFA, and animal user fee programs. Please see the Federal Register notices...
FY2025 Medical Device Fees
Please see below information regarding FY2025 Medical Device User Fees which go into effect 1 October 2024. Keep in mind, the FY2025 facility user fee must be paid prior to processing your annual renewal during the renewal period Oct 1 – Dec 31. FY2025 Medical Device...
Product Imports Into the U.S That Contain Meat and Poultry: Maneuvering the Process?
Product Imports Into the U.S That Contain Meat and Poultry: Maneuvering the Process? By Ronnie Dunn, EAS Consulting Group Independent Consultant Importing meat, poultry, and egg products into the U.S. can be a daunting and confusing task. There are numerous...
AHPA Claims Substantiation Tools and Trends
AHPA Claims Substantiation Tools and Trends Presented by Paula Brock, Ph.D., MCSI This is a portion of a webinar produced by the American Herbal Products Association (AHPA) originally held July 9, 2024. Enter Your Information to Watch Now By clicking submit above, you...
Ensuring Food Safety from the farm to the table is crucial. How inspections and audits play a key role.
Ensuring Food Safety from the farm to the table is crucial. How inspections and audits play a key role. by Don Abbott, EAS Consulting Group Independent Consultant Published in Food Safety...
USDA Releases Updated Guideline to Strengthen Substantiation of Animal-Raising and Environment-Related Claims on Meat and Poultry Labels
WASHINGTON, August 28, 2024 – The U.S. Department of Agriculture (USDA) announced today the availability of an updated guideline that makes recommendations to strengthen the documentation that supports animal-raising or environment-related claims on meat or poultry...
HDA 2024 Traceability Seminar: What to Expect From FDA Inspections
In an interview with Pharma Commerce Editor Nicholas Saraceno, Jeb Hunter, Senior Regulatory Consultant, EAS Consulting Group, discusses the on “What to Expect When They’re Inspecting: FDA Inspections on DSCSA Compliance” breakout session at the 2024 HDA...
Drug and Device August 2024
FDA issued a proposed administrative order to amend the requirements for internal analgesic, antipyretic, and antirheumatic drug products for OTC human use, as currently described in OTC Monograph M013. The proposed change will require the addition of a warning to the...
FDA Announces Proposed Rule to Require Submission of Tracking Numbers for Imports of E-Cigarette Products
Recently, FDA and the Department of the Treasury are announcing a proposed rule that would require an importer to submit the FDA-issued Submission Tracking Number (STN) of Electronic Nicotine Delivery System (ENDS) products into the electronic imports system operated...
How to Build an Achieving Team
How to Build an Achieving Team By Eiman Raouf, EAS Senior Regulatory Consultant Upon entering various organizations, scenarios often unfold where operational efficiency falls short. Quality lapses in production, a surge in customer grievances, or perpetual crisis...
Durbin Introduces Legislation To Improve Safety And Ensure Transparency Of Dietary Supplements
9 Out Of 10 American Adults Support Listing Requirements For Dietary Supplements WASHINGTON – U.S. Senate Majority Whip Dick Durbin (D-IL) today introduced the Dietary Supplement Listing Act of 2024, legislation to require dietary supplement manufacturers to list...
FDA Announces Registration and Listing Updates: The Release of New Discontinuation/Relisting Features in Cosmetics Direct
Cosmetics Constituent Update Today the U.S. Food and Drug Administration (FDA) announced the release of the following enhancements to help industry keep their registration and listing submissions up to date: the availability of two new features in Cosmetics Direct,...
Drug and Device July 2024
FDA has sent notices to drug establishment registrants reminding OTC hand sanitizer manufacturers who entered the market solely to provide hand sanitizer during the COVID-19 public health emergency, that beginning FY2025 OMUFA facility user fees will become mandatory....
FDA Enhances Tobacco Retailer Inspection Database
This week, FDA’s Center for Tobacco Products updated the presentation of information about tobacco compliance check outcomes on the FDA website and expanded the information available. The newly-named Tobacco Compliance Check Outcomes database provides an enhanced user...
Import Refusals – “Don’t Panic”
Import Refusals – “Don’t Panic” By Mark Moen, EAS Consulting Group Senior Regulatory Consultant Products imported into the US may be assessed and inspected by US Customs and Border Protection (CBP), the Food and Drug Administration (FDA), and US Consumer Products...
Drug and Device June 2024
Final reminder that CARES Act drug amount reports for 2023 are due by 31 July 2024. If you need assistance with this process, reach out to Victoria Pankovich for support. The FDA announced the availability of a final guidance for industry titled “Facility Readiness:...
FDA Releases Update of Priority Guidance Topics for Foods Program
Earlier this year, the FDA released the draft and final guidance topics that are a priority for the agency’s Foods Program to complete during 2024. Since January, the FDA has issued the following guidances that were on the list: New Dietary Ingredient (NDI)...
Product Claim Substantiation
Product Claim Substantiation By Jay Ansell, EAS Independent Consultant Substantiating a product claim is required by U.S. law and can help brands avoid regulatory action or provide an effective defense in litigation. For example, in April 2023, the US Federal Trade...
Drug and Device May 2024
Final reminder that OMUFA facility user fees are due 1 June 2024. Per MoCRA, responsible persons are required to submit any serious adverse event reports to the FDA within 15 business days of receiving such report. Cosmetic labels are required to include a domestic...
Ronnie Dunn
Ronnie Dunn was selected as the first Director of the United States Department of Agriculture (USDA) Food Safety and Inspection Service (FSIS) International Liaison Office in Beijing, China from July 2019-2022. In this role, Dunn shared best practices and promote the...
Jay Ansell
A senior safety and regulatory professional with extensive experience leading global Safety and Regulatory Affairs initiatives, Dr. Ansell founded Bellevue Toxicology LLC to provide comprehensive human safety science and regulatory services to the Personal Care...
Quality Management System (QMS) Documentation
Quality Management System (QMS) Documentation By Jeff VanderHoek, EAS Independent Consultant The design and manufacturing of medical devices requires a high level of quality and reliability to ensure patient safety and regulatory compliance. The Quality Management...
Omar Oyarzabal, Ph.D.
Senior Consultant for Food Services, Omar Oyarzabal, Ph.D. works directly with EAS’ Senior Director for Food Consulting Services to assure client satisfaction and project management. He develops food safety protocols per FSMA and HACCP regulations and facilitate...
Drug and Device April 2024
FDA announced the fiscal year 2024 OTC-Monograph Drug User Fee rates in the Federal Register Vol 89, No. 62 published 29 March 2024. These fees cover FDA’s FY2024 which runs from October 2023 through September 2024. The facility fees for FY 2024 are due on 3 June...
Tim Kapsala
Tim Kapsala works with EAS pharmaceutical clients to identify compliance gaps and deficiencies through audits and mock-FDA inspections, preparing reports documenting significant observations and providing recommendations to be used in remediation activities....
US label and EU label: food labeling in comparison
US label and EU label: food labeling in comparison Gisela Leon, EAS Consulting Group Independent Consultant Interviewed for Industrie- und Handelskammer Hannover April 4, 2024 Published in German Within the European Union, food labeling is clearly regulated. In the...
Control of Cronobacter in Dairy Dryer and Packaging Rooms
Control of Cronobacter in Dairy Dryer and Packaging Rooms By Beth Koenig, EAS Independent Consultant What Is Cronobacter and Why Should We Be Concerned About It? Cronobacter, a genus of bacterial pathogens in the Enterobacteriacea family is of considerable concern in...
FY2024 OMUFA User Fees Announced by FDA
FDA announced the fiscal year 2024 OTC-Monograph Drug User Fee rates in the Federal Register Vol 89, No. 62 published 29 March 2024. These fees cover FDA’s FY2024 which runs from October 2023 through September 2024. The facility fees for FY 2024 are due on 3 June...
FDA Issues Draft Guidance on New Dietary Ingredient Notification Master Files for Dietary Supplements
Today, the U.S. Food and Drug Administration (FDA) is announcing the availability of a draft guidance for industry titled “New Dietary Ingredient Notification Master Files for Dietary Supplements.” This draft guidance responds to the dietary supplement industry’s...
Eiman Raouf
Eiman Raouf is an accomplished food science leader with a big-picture understanding of the industry spanning product development, manufacturing, quality, food safety, operations, sustainability, and regulatory compliance developed over a 23-year career. Eiman has a...
FDA Issues Import Alert for Food Products with Chemical Contaminants Including PFAS
On March 20, 2024, the US FDA issued a new import alert for human food products with detectable levels of chemical contaminants that may present a safety concern to human health. The Import Alert 99-48, Detention without Physical Examination of Foods Due to Chemical...
FDA Launches Searchable Tobacco Products Database
Database Lists Tobacco Products—Including E-Cigarettes—That May Be Legally Marketed Today, FDA launched the Searchable Tobacco Products Database, a new user-friendly list of tobacco products—including e-cigarettes—that may be legally marketed in the United States. The...
Drug and Device March 2024
Reminder that CARES volume 2023 reports are due no later than 1 July 2024. Highlighted Guidance Documents Reporting Amount of Listed Drugs and Biological Products Technical Conformance Guide FDA is issuing this Technical Conformance Guide to assist registrants of drug...
Reassessment of EMPs
Reassessment of EMPs: Have You Identified the Correct Sampling Locations to Protect Your Product? An EAS Complimentary Webinar Presented by Rocelle Grabarek, EAS Independent Consultant March 27, 2024 • 1pm (Eastern) 1.5 hours Many of the environmental monitoring...
FDA Proposed Rule on “Labeling Requirements for Approved or Conditionally Approved New Animal Drugs”
FDA proposes to revise the existing regulations regarding the content and format of labeling for approved or conditionally approved new animal drugs to provide for a more comprehensive set of requirements in one location in the Code of the Federal Register (CFR)....
USDA Finalizes Voluntary “Product of USA” Label Claim to Enhance Consumer Protection
USDA today published its “Product of USA” final rule, which will allow voluntary “Product of USA” or “Made in the USA” label claims to be used on meat, poultry, and egg products only when they are derived from animals born, raised, slaughtered, and processed in the...
Jeff VanderHoek
Jeff VanderHoek is a Medical Device RA/QA professional with over 30 years of experience in developing Quality Systems that meet or exceed FDA and ISO requirements and Regulatory Submissions that achieve their intended purpose. He has hosted numerous FDA and Notified...
Are You Ready for the Unspoken Challenges of FSMA 204?
Are You Ready for the Unspoken Challenges of FSMA 204? By Norman Alayan, EAS Independent Consultant “The FDA final rule on Requirements for Additional Traceability Records for Certain Foods (Food Traceability Final Rule) establishes traceability record-keeping...
FDA Announces Release of First Final Guidance Section for NDIN – NDI Notification Procedures and Timeframes
The FDA recently announced final guidance for industry on “Dietary Supplements: New Dietary Ingredient Notification Procedures and Timeframes.” This guidance is intended to help manufacturers and distributors of new dietary ingredients (NDIs) and dietary supplements...
Thomas Savage
Thomas Savage served for 36 years with FDA in various roles. He began his agency career as a chemist in the FDA Los Angeles District Laboratory, retiring in 2010 as a senior policy advisor in the International Compliance Branch at CDER. After leaving the agency, he...
Drug and Device February 2024
The FDA has issued a final Guidance Document to assist drug establishment registrants with understanding the CARES Act reporting requirements. Reporting Amount of Listed Drugs and Biological Products Under Section 510(j)(3) of the FD&C Act guidance offers very...
Media Spotlight: EAS Consultant John Bailey
John Bailey, an EAS consultant and the former director of FDA’s Office and Cosmetics and Colors, shared his thoughts with KFF Health News on FDA’s Plan to Ban Hair Relaxer Chemical Called Too Little, Too Late. Read...
FDA Issued a Final Guidance Document on CARES Act Reporting Requirements
The FDA has issued a final Guidance Document to assist drug establishment registrants with understanding the CARES Act reporting requirements. Reporting Amount of Listed Drugs and Biological Products Under Section 510(j)(3) of the FD&C Act guidance offers very...
FDA Publishes Revised Draft Introduction and Appendix to the Preventive Controls for Human Food Guidance
The FDA has released a revised draft Introduction and Appendix 1 to the multi-chapter draft guidance for industry titled “Hazard Analysis and Risk-Based Preventive Controls for Human Food: Draft Guidance for Industry” (PCHF Draft Guidance). The changes...
FDA Issues Final QMSR Regulation and FAQ
The Food and Drug Administration (FDA) issued a final rule on January 31, 2024, to amend the medical device current good manufacturing practice (CGMP) requirements of the Quality System (QS) regulation defined in 21 CFR Part 820 to harmonize and modernize the...
FDA Issues Revised Draft Guidance for Topical Ophthalmic Drug Products
The FDA has issued a draft guidance document discussing Quality Considerations for Topical Ophthalmic Drug Products intended for topical use in and around the eye. This is an update to the draft guidance of the same name released in October 2023. The guidance...
Beth Koenig
Beth Koenig is passionate about food safety and has experience in a variety of food processing environments with emphasis on dairy processing. She has successfully developed, implemented and maintained quality and food safety management systems to meet stringent...
The Importance of the Medical Device Single Audit Program (MDSAP) and EU MDR Lead Auditor Certifications
The Importance of the Medical Device Single Audit Program (MDSAP) and EU MDR Lead Auditor Certifications By Kevin Walls, MBA, EAS Independent Consultant The Medical Device Single Audit Program (MDSAP) is a certification program many medical device manufacturers are...
Drug and Device January 2024
Reminder that per the CARES Act of 2020, drug establishments are required to report NDC volumes annually. This applies to all facilities that manufacture, prepare, propagate, compound, or process drugs for commercial distribution in the United States. Although the FDA...
Upcoming FDA Food Guidance Materials for 2024
On January 25, 2024, the U.S. Food and Drug Administration’s Foods Program posted a list of new / updated food regulations it plans to publish in 2024 and beyond. These include: Amendments to Registration of Food Facilities February 2024. The proposed rule would make...
FDA Announces Draft Guidance on Good Manufacturing Practice for Active Pharmaceutical Ingredients Used in Veterinary Drug Products
The FDA has announced the availability of a draft guidance for industry #286 (VICH GL60): “Good Manufacturing Practice for Active Pharmaceutical Ingredients used in Veterinary Medicinal Products.” This draft guidance has been developed by the International Cooperation...
Cosmetic Safety Substantiation
MoCRA, effective December 2023, requires safety substantiation. It amends the FD&C Act. MoCRA mandates maintaining safety records, facility registration, product ingredient registration, and reporting serious adverse events. This seminar, with insights from experts, will overview cosmetic safety assessment.

Norman Alayan
Norman Alayan has extensive experience in the fields of Quality Assurance, R&D, Production, Operations, Auditing, Consulting, and Training in Management Systems (Quality, Food Safety/HACCP, OH&S, Environment) in food manufacturing industries managing company’s...
Shaping the Future: Revolutionizing Food Safety and Quality
Shaping the Future: Revolutionizing Food Safety and Quality By Vera K. Petrova Dickinson, EAS Independent Consultant In the ever-evolving landscape of the food industry, the quest for ensuring Food Safety and Quality (FSQ) faces both new challenges and age-old...
Drug and Device December 2023
With the requirement for Cosmetic Responsible Persons to report Serious Adverse Events to FDA beginning 29 December 2023, the agency has recommended using the current Form 3500A that is downloadable and fillable at MedWatch: The FDA Safety Information and Adverse...
FDA Issues Updated Instructions for Serious Adverse Event Reporting for Cosmetic Products
Today, the U.S. FDA has provided an update on its ongoing activities related to serious adverse event reporting mandated by the Modernization of Cosmetics Regulation Act of 2022 (MoCRA), which are enforceable starting December 29, 2023. A responsible person is...
FDA Announces Availability of Draft Supplemental Guidance on Menu Labeling
The U.S. Food and Drug Administration (FDA) is announcing the availability of a draft guidance for industry entitled Menu Labeling: Supplemental Guidance for Industry (Edition 2). To respond to frequently asked questions regarding menu labeling requirements, the draft...
Rocelle Grabarek
Dr. Rocelle C. Grabarek has over 25 years of food safety experience in a broad range of human, infant formula, and pet food industry. She received her M.S. and Ph.D. in Food Science from the University of Georgia and has developed and conducted customized training in...
Drug and Device November 2023
Reminder that 31 December is the deadline for submitting your drug registration renewal, medical device establishment registration renewal and drug listing certification. If NDC listing blanket certification is not submitted, each individual SPL file will need to be...
Armen Asatryan
Dr. Armen Asatryan is an experienced clinical research and development leader trained as a physician-scientist with double board certifications (internal medicine and preventive medicine) and more than 25 years of combined experience in clinical medicine, public...
FDA Issues Compliance Policy for Cosmetic Product Facility Registration and Cosmetic Product Listing
Today, the U.S. Food and Drug Administration (FDA) announced its intent to delay enforcement of the requirements for cosmetic product facility registration and cosmetic product listing requirements under the Modernization of Cosmetics Regulation Act of 2022 (MoCRA)...
Remote Interactive Evaluations of Drug Manufacturing and Bioresearch Monitoring Facilities
FDA has issued a Draft Guidance “Remote Interactive Evaluations of Drug Manufacturing and Bioresearch Monitoring Facilities”. Comments may be made to Docket number FDA-2023-D-4416 by 26 December 2023. This draft guidance describes how the FDA will request and conduct...
Review of Food Safety Needs to Export from the USA
Review of Food Safety Needs to Export from the USA By Omar Oyarzabal PhD., Senior Consultant for Food Services Exporting foods from the USA to other countries, such as Canada and those in the EU block, has been a lucrative option for some segments of the food...
MoCRA: Facility Registration and Product Listing for Cosmetics
The Clock is Ticking: Are you Ready for Facility Registration and Product Listing under MoCRA? An EAS Complimentary Webinar Presented by John and Catherine Bailey, EAS Independent Consultants and Victoria Pankovich, Manager of Regulatory Services The new requirements...
Drug and Device October 2023
The FDA published a new Guidance Document – Alternative Tools: Assessing Drug Manufacturing Facilities Identified in Pending Applications which articulates the agency’s evolving policy which has grown from the Pandemic era use of alternative tools in evaluating...
Kevin Walls, MBA
Kevin Walls works with a domestic and international client base on regulatory issues, compliance, submissions, and training for the Medical Device and IVD Industries. He has expert knowledge of US FDA, Health Canada and the European Medical...
MoCRA Preparations Should be in Progress
As the cosmetic facility registration and product listing requirement date approaches (29 December 2023), companies needing to register their facility, or list cosmetic products should be gathering the information required to submit to FDA. For facilities that need to...
Tim Penne
With over 27 years of experience, I am a skilled and accomplished Analytical Chemist in the Pet Food and Food safety industries. As a executive, I oversaw major analytical divisions across pet food, animal feed, food, agricultural, and environmental industries-...
Don Abbott
Don is a 3rd generation food chemist who has worked as a Nutritional Biochemist developing food in 62 countries. Don has also performed over 1,000 food safety audits globally. Over the last 10 years, Don’s food safety and regulatory consulting has assisted facilities...
A Decade of Vigilance: FDA’s Battle Against Imported Foodborne Illness
A Decade of Vigilance: FDA’s Battle Against Imported Foodborne Illness By Don Abbott, EAS Independent Consultant Introduction Over the past decade, the Food and Drug Administration (FDA), particularly its Center for Food Safety and Applied Nutrition (CFSAN), has been...
The FDA Has Updated the Food Traceability Frequently Asked Questions on the Website
On September 28, 2023, the U.S. Food and Drug Administration (FDA) has updated its Food Traceability Frequently Asked Questions (FAQ) to provide additional information about this rule. The Food Traceability Final Rule was published in November 2022 and requires the...
FDA Issues New Guidance for Allergen Management and Control
On September 26, 2023, US FDA issued an updated Draft Guidance for Industry: Hazard Analysis and Risk-Based Preventive Controls for Human Food. This new guidance material includes a new chapter specific to allergens. Key take-aways include: Establish and Implement a...
Charles Eirkson
Charles Eirkson, a noted expert in the areas of environmental impact assessments and hazard reviews of veterinary pharmaceutical and food/animal feed products, comes to EAS after a distinguished career at FDA where he worked closely in the development and...
Drug and Device September 2023
Reminder that FY2024 GDUFA payments are due 2 October 2023. The FDA does allow a 20 calendar day grace period, after which arrears lists will be published with the names of companies that have failed to make payment. Failure to pay required user fees by this deadline...
Food labeling: EU versus USA
Food labeling: EU versus USA Gisela Leon, EAS Consulting Group Independent Consultant Interviewed for Industrie- und Handelskammer Hannover September 21, 2023 Published in German There are uniform EU-wide regulations on how food should be labelled in general and what...
FDA Issues Draft Guidance on Registration and Listing of Cosmetic Product Facilities and Products
On Friday 15 September 2023, the FDA issued a draft guidance shedding light on the much-anticipated MoCRA registration and listing requirements. The guidance, titled “Registration and Listing of Cosmetic Product Facilities and Products” gives an overview of the...
Theresa Whitemarsh
Theresa Whitemarsh is a seasoned food industry leader with extensive experience in FDA and USDA labeling regulations, substantiation of compelling and compliant product claims, certifications like Kosher, Organic, and non-GMO, and management of regulatory-driven...
“Tree Nut” Allergy; Is It a Misnomer?
“Tree Nut” Allergy; Is It a Misnomer? By Lisa Zitiello, EAS Consulting Group, Independent Consultant Food safety professionals across the globe will agree that one key safety factor in food production today is the avoidance of allergen cross-contact. This is closely...
FDA Extends Comment Period for TPMP Proposed Rule
On March 10, the U.S. Food and Drug Administration published a proposed rule titled, Requirements for Tobacco Product Manufacturing Practice. FDA is extending the proposed rule’s comment period by an additional 30 days to allow people additional time to submit...
Drug and Device August 2023
We will be entering the establishment renewal and product listing certification period soon. As your regulatory support partner, EAS is here for any questions you may have. If you wish for us to handle the annual requirements, please email Victoria Pankovich to get...
Federal Judge Strikes Down FDA Regulation of Premium Cigars
In a major win for the premium cigar industry, Judge Amit Mehta of the U.S. District Court for Washington D.C. has vacated FDA’s deeming regulations for premium, hand-rolled cigars. As a result, the regulations introduced by the FDA in 2016 do not apply to cigars that...
Beverage Manufacturing
What’s Hot in the Beverage Industry? A look at trends and regulations for beverage manufacturing Presented by Omar Oyarzabal, Ph.D., EAS Consulting Group and Alex Brandt, Ph.D. Certified Group The beverage industry is thriving. From sports recovery to hydration, food...
Status of Regulatory Compliance of Establishments Under USDA FSIS Jurisdiction
Status of Regulatory Compliance of Establishments Under USDA FSIS Jurisdiction By Omar A. Oyarzabal, PhD, EAS Senior Consultant The U.S. Department of Agriculture’s Food Safety and Inspection Services (USDA FSIS) collects information on compliance under its...
FDA Releases New Guidance on Cosmetic Facility and Product Registration
On August 7, FDA issued a new draft guidance on the registration and listing of cosmetic product facilities and products, which is mandated by the Modernization of Cosmetic Regulations Act of 2022 (MoCRA). This guidance is not legally enforceable, but it provides...
John Mwangi
John Mwangi, Ph.D., is a Senior Food Scientist with extensive experience in international regulatory affairs, quality management, and FSMA Implementation. His work experience includes Starbucks Coffee Company, managing Technical Scientific Regulatory Affairs for...
Fred Lochner
Mr. Lochner joined EAS Consulting Group after a distinguished career at the FDA where he performed domestic and international supplier and internal audits of drug and API manufacturers and testing laboratories for compliance with FDA regulations. He also performed...
Drug and Device July 2023
Thinking about an FDA gap assessment this year? Contact EAS now to schedule your 2023 audit. Schedules toward the end of the year tend to book up suddenly, now is the time to lock in your audit, whether it be onsite or virtual. We would be happy to prepare an audit...
Congress Seeks Input on CBD Regulation by August 18
On July 27, 2023, the U.S. Congress requested information to guide the assessment of the potential for a regulatory pathway for hemp-derived Cannabidiol (CBD) products with the goal to prioritize consumer safety and bring certainty to the U.S. market. Since the...
Labstat Inc., A Certified Group Company, Highlights Need for Enhanced Cannabis Regulations to Protect Consumers
TORONTO, Aug. 2, 2023/PRNewswire/ — As Canada approaches the 5th anniversary of cannabis legalization, Labstat Inc., a leader in tobacco/nicotine, cannabis, hemp and NHP laboratory testing and research & development, underscores the need for more robust...
Food Safety Net Services (FSNS) Moves to New Laboratory in Logan, Utah
LOGAN, Utah, July 26, 2023 /PRNewswire/ — Food Safety Net Services (FSNS), A Certified Group Company, has moved to a new state-of-the-art laboratory with increased capabilities in Logan, Utah. The lab is equipped with the latest scientific instruments to provide...
Labstat Inc., A Certified Group Company, Opens New Laboratory in Greensboro, North Carolina, Strengthening its Global Network
KITCHENER, Ontario, July 20, 2023 /PRNewswire/ — Labstat Inc., an industry-leading provider of tobacco/nicotine, cannabis/hemp testing and research services, is excited to announce the opening of a new laboratory in Greensboro, North Carolina. This significant...Protected: Claims Substantiation
Password Protected
To view this protected post, enter the password below:

Vera K. Petrova Dickinson, Ph.D.
Dr. Vera K. Petrova Dickinson fosters data-driven, integrated approaches to ensure the safety and integrity of the food system. This includes helping businesses and organizations of all types to improve their strategies and processes to prevent food-borne issues...
Biofilms and Healthcare
Biofilms and Healthcare By David W Koenig, PhD., EAS Consulting Group Independent Consultant Healthcare facilities — such as hospitals, nursing homes and outpatient facilities — are opportunistic locations for acquiring secondary infections unrelated to a...
Drug and Device June 2023
The FDA has published a new Guidance Document Assessing User Fees Under the Generic Drug User Fee Amendments of 2022 which explains in great detail the GDUFA program user fee obligations. GDUFA III made slight changes to the user fee structure. (API facility fees...
Review of the Foreign Supplier Verification Program’s Guidance for Industry
Review of Foreign Supplier Verification Program’s Guidance for Industry An EAS Complimentary Webinar Presented by Tim Lombardo, Senior Director for Food Consulting Services and Omar Oyarzabal, Ph.D., Senior Consultant The Food and Drug Administration has released the...
Csilla Goldsmith
Csilla Goldsmith has worked in Regulatory Affairs and Quality Assurance in the flavour industry for the past 21 years and has a deep understanding of the Canadian Food and Drug Regulations. Her expertise includes extensive knowledge in the Canadian Organic...
The Food Safety Language
By Rafael Olivares, EAS Independent Consultant A career in food safety is not an easy one. It is a stressful profession that requires dedication and a commitment to learn continuously and to teach, to communicate effectively to an audience, in most cases, obstinate,...
Ensuring Compliance with FDA Guidelines for Glycerin Identity Testing
A recent FDA warning letter concerning identity testing and contaminated glycerin highlights the significance of a recurring potential public health hazard. After several lethal poisonings worldwide, it is apparent that high-risk drug components contaminated with...
Drug and Device May 2023
As we approach June, keep in mind that drug establishment registrations and NDC listings that have had any changes to their information must update their files with the FDA within 30 days. Failing that, the agency expects updates to be submitted the JUNE or December...
TMA 2023 Introduction to FDA Tobacco Product Manufacturing Practices
Introduction to FDA Tobacco Product Manufacturing Practices Dean Cirotta’s presentation during the TMA 2023 Annual Meeting April 17-18, 2023 Leesburg,...
FDA Proposes Long-Awaited Tobacco Product Manufacturing Practices
By Shelly Blackwell, Senior Director for Dietary Supplement and Tobacco Services Over 12 years after the FDA began regulating tobacco products, the long-anticipated Tobacco Product Manufacturing Practices (TPMP’s) have been proposed. The new requirements were proposed...
Drug and Device Corner April 2023
Reminder that the FDA has announced the 2023 OMUFA user fee rates, please see the Over-The-Counter Monograph Drug User Fee Program (OMUFA) website for full details. Monograph Drug Facility (MDF) Facility fee: $26,153; Contract manufacturing Organization (CMO) Facility...
Certified Group Welcomes Nick Buschur as President of Food & Beverage Business Unit and Tim Daniels as Chief Information Officer
Certified Group Welcomes Nick Buschur as President of Food & Beverage Business Unit and Tim Daniels as Chief Information Officer Executive Hires to Accelerate Growth and Strengthen Company’s Position in Laboratory Testing Services SAN ANTONIO, April 11, 2023 —...
Charles Giambrone
Charles Giambrone has strong technical and managerial skills in private sector Food Safety & Healthcare Q.A. and Plant Hygiene-Sanitation. He has a proven record of accomplishment in Food-HACCP and SSOPs in processing markets and is known for his expertise in...
Claims Substantiation for Dietary Supplements: A Scientific and Legal Approach
By Paula Brock, Ph.D., MSCI, EAS Independent Consultant It is required in the United States that manufacturers and other advertisers of dietary supplements such as distributors, retailers, and endorsers, have adequate substantiation that the claims made about their...Meeting USDA Federal Food Regulatory Requirements
USDA Consulting On-Demand Webinar Hear from the Experts: Meeting USDA Federal Food Regulatory Requirements Learn how to expand your operation without sacrificing safety. How are you preparing for the long-term growth of your business? Whether you’ve recently...
FDA Issues Draft Guidance on Dietary Guidance Statements in Food Labeling
FDA’s latest draft guidance document on Dietary Guidance Statements in Food Labeling shares FDA’s current thinking on label, labeling and marketing statements that aims to help Americans make informed food choices. FDA has long actively supported consumer awareness of...
FDA Has Stopped Accepting Submissions to the Voluntary Cosmetic Registration Program (VCRP)
Contrary to the expectation in the cosmetic regulatory space, the FDA has stopped accepting submissions to the voluntary cosmetic registration and listing program and will not be transferring this data to the program the Agency is establishing for the mandatory...
An Introduction to Proposed FDA Tobacco Product Manufacturing Practices (TPMPs)
An Introduction to Proposed FDA Tobacco Product Manufacturing Practices (TPMPs) An EAS Complimentary On Demand Webinar Presented by Dean Cirotta, EAS Consulting Group President and Shelly Blackwell, EAS Consulting Group Senior Director for Dietary Supplement and...
Drug and Device Corner March 2023
The FDA has announced the 2023 OMUFA user fee rates, please see the Over-The-Counter Monograph Drug User Fee Program (OMUFA) website for full details. Monograph Drug Facility (MDF) Facility fee: $26,153; Contract manufacturing Organization (CMO) Facility Fee: $17,435....
An Introduction to Upcoming Modernization of Cosmetics Regulation Act (MoCRA)
An Introduction to Upcoming Modernization of Cosmetics Regulation Act (MoCRA) An EAS Complimentary On Demand Webinar Presented by John and Catherine Bailey, EAS Independent Consultants. The Modernization of Cosmetics Regulation ACT (MoCRA) of 2022 was signed into law...
FDA Calls for Enhanced Safety Measures in Letter to Powdered Infant Formula Industry
On March 8, 2023, US FDA issued a “Call to Action” letter to all powdered infant formula manufacturers, packers, distributors, exporters, importers, and retailers in order to help protect our most vulnerable population. The FDA has reviewed conditions during recent...
USDA FSIS Announces Draft of New Requirements for Voluntary Use of ‘Made in the USA’ Labels
The US Department of Agriculture Food Safety Inspection Service (USDA FSIS) announced draft of a proposed change to voluntary labeling requirements for meat, poultry and eggs that use the claim ‘Made in the USA’ or ‘Product of the USA.’ Under the proposed change, use...
Developing a Food Safety Culture: Challenges and Best Practices
Developing a Food Safety Culture: Challenges and Best Practices By Angie Surtani, EAS Consulting Group Independent Consultant Food safety culture is a journey that requires significant hard work, time, effort, persistence, and commitment for fruition. I consider food...
Sandeep Modi
Sandeep P. Modi, PhD, is experienced in ICH guidelines and developing CMC regulatory strategies for global applications (IND/CTA/NDA/MAA), and is successful in the timely preparation of CMC documents for global regulatory submissions that address development and...
Kevin Ragland
Dr. Ragland is currently the Associate Director of the Tennessee STEM Education Center at Middle Tennessee State University and an Associate Professor of Animal Science. Prior to joining MTSU in 2022, Dr. Ragland was employed as the Senior Principal Nutrition...
David Koenig
Dr. David W Koenig has over 36 years of experience in the Life Sciences (Microbiology and Skin Biology) with government labs (NASA), private industry R&D, and academia. Working for NASA, Betz PaperChem, Kimberly Clark Global, and Kimberly Clark Professional have...
New Dietary Supplement Ingredients Directory Launched by FDA
FDA has launched an on-line directory that lists 27 articles used in dietary supplements enabling quick references to any information FDA currently has pertaining to these articles and any actions taken such as constituent updates and warning letters. Additionally,...
Drug and Device Corner February 2023
The FDA sent email reminders in February re mandatory CARES annual reporting for drug establishments, which are due now. CARES volume reporting has been an annual requirement since passing of the CARES Act in 2020. If you have not already submitted your 2020 and 2021...
USDA Announces National Organic Program (NOP) previewed the Strengthening Organic Enforcement (SOE) Final Rule
Earlier this year, USDA announced the National Organic Program (NOP) Strengthening Organic Enforcement (SOE) final rule (https://www.federalregister.gov/documents/2023/01/19/2023-00702/national-organic-program-nop-strengthening-organic-enforcement). This rule is an...
FDA Seeks Maximum Allowable Civil Monetary Penalties Against Unlawful E-Liquid Manufacturers
In a first for FDA’s Center for Tobacco Products, Civil Monetary Penalty complaints have been filed against four manufacturers of e-liquids for violation of the Food Drug and Cosmetic Act (FD&CA) by selling tobacco products without an FDA marketing order. FDA had...sandbox – survey

FDA Issues 2022 Food Code
Late last year, the FDA issued the 2022 edition of the FDA Food Code providing guidance to state and local authorities and retailers to help mitigate foodborne illness risks at retail and provide a uniform set of national standards for retail food safety. The 2022...
FDA Enforcement Discretion for Certain Qualified Health Claims and Cocoa Flavanols
FDA has indicated enforcement discretion for certain qualified health claims regarding the consumption of cocoa flavanols in high flavanol cocoa powder and a reduced risk of cardiovascular disease for conventional foods. With “very limited credible scientific...
FDA Proposes Restructuring Food Safety Efforts into New Human Foods Program
In a January 31, 2023 announcement, FDA proposed a vision to restructure the oversight of food safety and informed consumer choices into a new organization which will be known as the Human Foods Program. As part of this restructuring, the Center for Food Safety and...
Process and Manufacturing Optimization to Minimize Inflation Impact on the Pharmaceutical Industry
Process and Manufacturing Optimization to Minimize Inflation Impact on the Pharmaceutical Industry By Ibrahim Khattab, EAS Independent Consultant Rising inflation rates, the Russian invasion of Ukraine and the associated rise in energy prices, and the numerous...
FDA Issues Final Guidance on GRAS Panel Best Practices
The U.S. Food and Drug Administration (FDA) has issued a final guidance titled Best Practices for Convening a GRAS Panel. Although in most cases general recognition of safety can be supported without convening a GRAS panel, this guidance provides information to those...
Divya Gowdar
An experienced Quality and Regulatory Professional, Divya has worked across multiple product portfolios in both large and small medical devices and combination product organizations. She offers a proven track record of successful contribution in the FDA cGMP/QSR, 21...
Drug and Device Corner January 2023
On 29 December 2022, the U.S. President signed H.R 2617 – the Consolidated Appropriations Act, 2023 into law which includes the Food and Drug Omnibus Reform Act. Of particular interest in this legislation for EAS drug and device clients, are the following: The...
Guidance for Industry: Homeopathic Drug Products
What You Need to Know The U.S. Food and Drug Administration has issued a final guidance on Homeopathic Drug Products describing the agency’s approach to prioritizing regulatory actions for homeopathic products posing the greatest risk to patients. The agency...
Importing: What Happens If Something Goes Wrong?
Importing: What Happens If Something Goes Wrong? By Nick Lahey, EAS Consulting Group Independent Consultant The U.S Food and Drug Administration (FDA) works hand and hand with U.S. Customs and Border Protection (CBP) with the import process of FDA-regulated...
Drug and Device Corner December 2022
We wish you all a very happy holiday season and prosperous new year. Reminder of the deadline of 31 December 2022 for the establishment registration and product listing renewal period. For those that have not already submitted their CARES Act reporting for FY2020 and...
What Not To Feed Your Dog
Originally posted on American Council on Science and Health By Jane Caldwell, Ph.D. We love our dogs and puppies. They are faithful, non-judgmental companions who delight in our presence. Many pet owners return this affection by feeding them treats. But some human...
Drug and Device Corner November 2022
Reminder that December is the deadline for the establishment registration and product listing renewal period. Kindly note the FDA has tightened up their validation process for drug listings. Although repack / relabeled listings have been submitted in the past without...
Rafael Olivares
Rafael Olivares is an accomplished food safety, regulatory, and quality assurance professional with more than twenty years of experience in the food industry. Olivares is considered a Regulatory and HACCP subject matter expert due to extensive experience in the...
Nick Lahey
Accomplished Commissioned Officer with the United States Public Health Service (Commander) with 20 years of Duty Station experience at the US Food and Drug Administration (FDA). An influential leader who acts as a strategic regulatory partner within cross-departmental...
Angie Surtani
Passionate and results-driven, Angie Surtani has over 25 years of experience in the food industry and deep functional expertise in Food Safety, Quality and Regulatory systems. She has a master’s degree in Food Science and Nutrition and is proficient in handling...
Jignesh Kahodariya
Jignesh Kahodariya is an independent consultant in areas such as GxP Compliance, Microbiology, Computer System Validation and Data Integrity, Pharmacovigilance, Supplier Qualification and Vendor Management, Internal and External Audits, Regulatory Agency Inspection...
Low Acid Canned Food and Acidified Foods – Current Topics for the Food Industry
EAS Consulting Group and partner organization and testing laboratory, Certified Laboratories, are co-presenting a complimentary webinar that will review the basic regulatory requirements of low acid canned foods and acidified foods …

How the Voluntary Qualified Importer Program (VQIP) Can Expedite Your US Imports
Most exporters of food products to the US are plagued by FDA import bottlenecks at the border. From paperwork to random and for-cause inspections, importing often feels like a waiting game. For everyone who has thought, Wouldn’t it be nice if FDA product imports could be expedited, this EAS Consulting Group, a Certified Group company, webinar discusses FDA’s “fast track” program for food imports called the Voluntary Qualified Importer Program (VQIP).

GDUFA III Targets New FDA Initiatives to Expedite Reviews and Inspections
FDA is pushing forward with new initiatives targeting enhancements to the Generic Drug User Fee Amendments (GDUFA) program as announced in a November 2021 commitment letter to industry. The current and third iteration of the GDUFA program, known as GDUFA III, enables...
Drug and Device Corner October 2022
As many are aware, FY2023 FDA user fees were pending authorization from Congress which did not happen until 30 September 2022. As a result, the user fees were announced in early October 2022. Please see Federal Register links and screen shots of fee tables below....
Listeria monocytogenes in Ice Cream and Frozen Novelties
Ice cream is often associated with childhood, special occasions, and moments of pleasure with family and friends. Everyone has special ice cream memories! However, memories of eating ice cream should not be associated with illness, hospitalization, or death.

Dietary Supplement Product Development for Commercial and Compliance Success
Dietary Supplement Product Development for Commercial and Compliance Success Dietary supplement product development requires diligent product design, qualification and launch strategies that position your products ahead of the competition. Innovation is key in this...Establishing an Effective Environmental Monitoring Program
Prevention of product contamination through an Environmental Monitoring Program is a requirement across FDA regulated industries. This ensures product safety and reduces the likelihood of an adverse event(s) for the consumer. An established EMP is required by FDA as part of a Good Manufacturing Practice (GMP) quality system.

Responding to an FDA Form 483 – Tips for Compliance Success
By Amy Scalin, M.S. Hearing from FDA often means one thing: The Agency has found issues with your operations that require a swift response and corrective actions. FDA uses numerous tools to notify firms of regulatory compliance enforcement. Most commonly, a firm will...
EAS Welcomes Shelly Blackwell as Senior Director for Dietary Supplement and Tobacco Services
EAS Consulting Group is pleased to welcome Shelly Blackwell to the EAS management team. As Senior Director for Dietary Supplements and Tobacco, she will be responsible for overseeing safety, submissions, and general regulatory intelligence under the purview of FDA,...
Developing Dietary Supplement Specifications – No “By Input” Here
Regulatory expert Steve Cammarn, Ph.D., discusses specifications testing for dietary supplement finished products. Use of “By Input” to verify the strength of a dietary ingredient or to exempt a finished product from testing is never acceptable.

Developing and Implementing a Supplier Qualification Program for Dietary Supplements
In this webinar, regulatory expert and EAS Independent Consultant Aisha Siddiqui explains what you need to know when developing a component supplier qualification program for dietary supplements.

Drug and Device Corner September 2022
As of 26 September 2022, the U.S. Congress has yet to pass the FDA user fees bill for FY2023 which begins 1 October 2023. EAS will keep our client based informed as this situation develops. EAS wishes to announce the retirement of Ms. Susan Crane, EAS Independent...
Carolyn Troiano
Carolyn Troiano has more than 40 years of experience in Computer System Validation (CSV) and compliance in the pharmaceutical, medical device, tobacco, and other FDA-regulated industries. She is currently an independent consultant advising companies on computer system...
Jane M. Caldwell, PhD
Jane M. Caldwell, PhD, has over 25 years diverse experience with analytical testing and R & D in food, pet food, water quality, poultry production, aquaculture, epidemiology, and molecular biology. She was formerly director of Merieux’s food testing lab in...
Christina Farnan
Christina Farnan is a highly regarded expert with over 25 years of senior management experience in food, dairy, poultry, infant formula manufacture and food chain safety management. Based in Ireland, she recently completed numerous training and guidance documents for...
New York High Court Rejects Talc/Asbestos Causation Testimony, Reaffirming Need for Scientific Dose Assessment
By William A. Ruskin, EAS Independent Consultant and William L. Anderson, former partner at Crowell & Moring This article is a revision of an article that first appeared in the Lexis Nexis publication Editor’s Note: Mr. Anderson is a former partner with...
Updates to Retail Program Standards Offers Flexibility
FDA recently released an updated 2022 edition of the Voluntary National Retail Food Regulatory Program Standards (Retail Program Standards) for both food service and food retail establishments. These standards give recommendations for designing, managing and...
Drug and Device Corner August 2022
Reminder that the establishment registration and product listing renewal period begins 1 October 2022. Contact Victoria Pankovich, vpankovich@easconsultinggroup.com if you would like EAS to handle this process for you. We can pre-schedule an appointment in October,...
Certified Group Welcomes Amanda Bosse as CEO
CEO transition to strengthen and accelerate the company’s growth by driving innovation in the lab testing market and expanding production capacity to better serve customers SAN ANTONIO, Aug. 25, 2022 /PRNewswire/ – Certified Group, a leading provider of testing and...
FDA Proposed Rule on Revising the National Drug Code Format
The much anticipated Proposed Rule on Revising the National Drug Code Format was announced in the FR Vol 87, No. 141 Revising the National Drug Code. The FDA is proposing a 12 digit uniform NDC number to replace the current format of 10 digits, which when exhausted...
Certified Group Partners with Groundswell Strategy to Strengthen Their Position as Food Safety Experts
SAN ANTONIO, August 17, 2022/PRNewswire/ — Certified Group, a leading provider of laboratory testing services for customers working in FDA and USDA-regulated markets, today announced a partnership with Groundswell Strategy. The partnership enables Certified...
Adverse Event Reporting for OTC Drug Products
By Susan Crane, EAS Senior Advisor for OTC Drugs and Labeling As part of its mission to protect the public health, the FDA monitors the safety of drug products marketed in the United States. This is accomplished through laws enacted by Congress as well as regulations...
Responding to an FDA Notice of Action
Timing is Everything When importing FDA-regulated products into the U.S., time is money. So, when you receive an FDA Notice of Action, a prompt and appropriate response will expedite what comes next. Nearly 15% of U.S. imports are FDA-regulated products. That amounts...
Guidance for Industry: Enforcement Policy for Providing an Acceptable UFI
What You Need to Know EAS food facility registration clients will remember during the 2020 renewal period, the FDA had sent reminders of the requirement per 21 CFR 1.232(a)(2), of a unique facility identifier (UFI) for every location registered with the agency as a...
Tim Bamiro
Tim Bamiro brings 25 years of food industry experience to the EAS team in various roles such as USDA commodity grader, quality assurance manager, production supervisor and food microbiologist. In addition, he has 13 years experience as a lead auditor to GFSI...
John B. Atwater, Ph.D.
John B. Atwater, Ph.D. has over 30 years of experience in analytical chemistry, quality assurance and healthcare product development. Dr. Atwater is currently the principal consultant at Ataqua Regulatory Services. Prior to that, he worked at USP for 19 years, where...
Drug and Device Corner July 2022
EAS bids a fond farewell to Mr. Bryan Coleman, and welcomes Mrs. Lisa El-Shall as our new Sr. Director Pharmaceutical and Medical Device Consulting Services. Lisa can be reached at lelshall@easconsultinggroup.com, 571-447-5504. The FDA has finalized the UDI draft...
How to Get Microbiological Projects and Shelf-life Testing Done Easily
An extra day of shelf-life could save billions of dollars in wasted food. A 2014 study discovered that $2.64 billion could be saved annually if food waste could be reduced by giving products an extra day of shelf-life. The USDA also discovered in a 2010 study that 31%...
FDA Expands Use of Remote Regulatory Assessments (RRA)
What You Need to Know FDA’s use of Remote Regulatory Assessments (RRA), originally developed during the COVID-19 health emergency, is being expanded. Announced in a Draft Guidance for Industry on July 22, 2022, FDA intends to use mandatory and voluntary RRAs as part...
Food Allergens and the FDA
Food Allergens, Contamination Risks, and the FDA Regulatory expert Steven Gendel discusses the many struggles of implementing an effective allergen control system. Nearly 20 years since the passage of the Food Allergen Labeling and Consumer Protection Act of 2004...
EAS Welcomes Lisa El-Shall as Senior Director, Drug and Device Services
EAS Consulting Group is pleased to welcome Lisa El-Shall to the EAS management team. As Senior Director for Drug and Device Services, she will be responsible for advising clients on FDA regulatory and GMP compliance matters related to pharmaceutical and medical device...
Drug and Device Corner June 2022
The FDA is tightening up the validation process in their NDC drug listing system CDER Direct. Prior to 2022, Relabelers and Repackers were able to list their products NDC/label without identifying the source NDC. As of 2022, the drug listing of a repackaged or...
Steve Martin
Steven Martin provides sanitation support through adoption and training of procedures for compliance to applicable FDA And USDA regulations. Prior to consulting he was the Senior Technical Specialist Fluid Foods for Delaval Cleaning Solutions. He has also provided...
Edyta Whelehan
Edyta Whelahan assists EAS clients with Food Safety and International Food Safety requirements per FDA, FSMA, HACCP, and BRC standards. She is proficient in meat, bakery items, retail food, juice, produce, and dairy. Edyta has a Master of Science from Nicolaus...
Environmental Monitoring Programs in Tablets and Capsules Magazine
EAS Consultant, Tamika Cathey, is featured in Tablets and Capsules magazine with an article on establishing an effective environmental monitoring program. Contamination is costly, with recalls and fines for problematic products in the hundreds of millions. EAS offers...
Botanical Drugs: What Might the Future Hold?
By Brad Douglass, EAS Consulting Group Independent Consultant The Food and Drug Administration’s approval of Fulyzaq (crofelemer), an anti-diarrheal drug for HIV/AIDS patients, was a first for an oral, prescription botanical drug. Prior to that approval, the topical...
EAS and Labstat Welcome New Incoming CTP Director, Brian A. King, Ph.D., M.P.H.
FDA’s Center for Tobacco Products (CTP) has announced the selection of a new CTP director, Brian A. King, Ph.D., M.P.H. Dr. King previously worked at the Centers for Disease Control and Prevention’s Office on Smoking and Health. Dr. King is charged with furthering...
How Would FDA’s Dietary Supplement Listing Act of 2022 Impact Your Business?
A bill before U.S. Congress proposes a new requirement that all dietary supplement products be electronically listed with Secretary of Health and Human Services. This move will enable the creation of a publicly available on-line registry in support of consumer...
The Importance of Testing Pet Food for Possible Contamination
The American Pet Products Association (APPA) estimates that 68% percent of U.S. households own a pet, according to the 2017-2018 National Pet Owners Survey. That means roughly 85 million families in the U.S. have a pet in their household. Many households treat their...
Drug and Device Corner May 2022
The passing of the CARES Act in 2020, built on the authorization given to FDA in 2012 by the Food and Drug Administration Safety and Innovation Act (FDASIA), to address the problem of drug supply disruptions and shortages. The agency released a draft guidance to...
Assume Nothing: What You Think You Know About the Cannabis Industry May Not Be True
An EAS and Food Safety News Complimentary Webinar. Assume Nothing: What You Think You Know About the Cannabis Industry May Not Be True Presented by Kathy Knutson, Ph.D., EAS Independent Consultant. The food industry is built on a history of Good Manufacturing...
Georgina Sofronas
Georgina Sofronas has over 25 years of Canadian Regulatory Affairs expertise at global top-tier companies such as SmithKline Beecham, Pfizer, Cadbury, Colgate-Palmolive and Estee Lauder where she has played leading roles in regulatory classification, claims...
William Ruskin
William Ruskin is an experienced attorney providing regulatory support and expertise in scientific disciplines including medicine, toxicology, epidemiology, drug efficacy, industrial hygiene, agronomy, toxicity, earth chemistry and hydrogeology. He has supported...
Joe Mitchell, Jr, Ph.D.
Joe Mitchell has extensive experience in Pharmaceutical, Dietary Supplement and Food Quality Management Systems. This thorough knowledge results in practical implementation of applicable cGMP regulations and compliance with federal requirements. Joe has served in...
Ibrahim Khattab, Ph.D.
Dr. Khattab has more than 30 years of pharmaceutical industry expertise, including eight years as the plant manager at Kuwait Saudi Pharmaceutical Industrial Company (KSPICO). In addition, he has provided consulting services to a number of pharmaceutical firms in the...
EAS Partner Laboratory Assist with Cosmetic Degradation Studies
EAS partner laboratory was mentioned in a recent article published in Beauty Independent on their partnership with a major brand to verify degradation of skincare ingredients. Micro Quality Labs, and all of the Certified Group, FSNS testing laboratories offer sound...
FDA Proposes Rules Prohibiting Menthol Cigarettes and Flavored Cigars
As has long been anticipated, FDA, on April 28, 2022, announced a proposed rule that would prohibit menthol as a characterizing flavor in cigarettes, and all characterizing flavors other than tobacco in cigars. Once finalized and implemented, FDA enforcement will...
Jennifer Kane
Jenifer Kane has 36 years of experience in the food industry, most recently as senior industry advisor with the International Food Protection Training Institute. Prior to that role, she was director of global quality auditing for the Kellogg Company. She previously...
CTP’s Draft Guidance on Analytical Testing Methods Validation and Verification for Tobacco Products
CTP’s Draft Guidance on Analytical Testing Methods Validation and Verification for Tobacco Products What You Need to Know Presented by Charlotte Peyton, EAS Independent Consultant. FDA’s December 2021 draft guidance on validation and verification testing methods used...
EAS Publishes Article on Packaging Challenges for FDA Industries in Tablets and Capsules
EAS Independent Consultant and expert in packaging solutions for FDA industries, Jim Goldman, wrote an article on quality considerations and mitigations of supplier and customer challenges that was published in Tablets and Capsules. EAS experts such as Jim help...
Steven M. Gendel, Ph.D.
Steven Gendel works to protect public health and to leverage safety and integrity systems to enhance success and sustainability. His experience includes over two decades as a scientist, risk assessor, and policy coordinator in the FDA Center for Food Safety and...
Establishment Registration and Drug Listing Still a Challenge for Some
By Susan Crane, EAS Independent Consultant It’s now been 5 years since FDA updated the regulations for establishment registrations and drug listings. Despite numerous on-line reference materials and tutorials available, it appears that some companies still have...
Phil Dazo
Phil Dazo is an experienced product developer with over 35 food industry experience with product development and launch of mayonnaises, dressings and sauces ambient, both refrigerated and frozen in the USA and abroad. Additionally, he is an expert in acid, acidified...
Environmental Impact Assessments are Required by FDA, NEPA
By Charles Eirkson, EAS Independent Consultant The National Environmental Policy Act (NEPA, 1969), requires that potential environmental impact of actions, e.g., approvals of new drugs, biologics, food additives, be addressed by the U.S. Food and Drug Administration...
Food Fraud Mitigations and Testing
EAS and Food Safety News Complimentary Webinar Food Fraud Mitigations and Testing Presented by EAS Senior Directors,Tim Lombardo, Food Consulting Services andMaged Sharaf, Ph.D., Labeling, Cannabis and Claims Services. Food Fraud is a global challenge, costing...
Betty Walker Collins
Betty Walker Collins has been an EAS consultant for nearly 12 years, assisting medical device clients with a variety of regulatory actions including seizures, consent decrees, depositions, regulatory letters, warning letters, civil money penalties, Section 518 recall...
Drug and Device Corner April 2022
We are 5 months out from the UDI / GUDID enforcement date of 22 September 2022 for Class I and unclassified devices, are your processes in place? Do you know what requirements are applicable to your operation? Helpful information can be found on FDA’s webpage GUDID...
U.S. FDA’s Latest Requirements for the 510(k)
U.S. FDA’s Latest Requirements for the 510(k) Presented by John Lincoln, EAS Independent Consultant. The majority of medical devices are cleared for marketing in the U.S. by the FDA under the 510(k) process, others are exempt, and some must go through the...
Good Auditing Practices, Making the Most of Your Internal Review
Regular and formal internal audits provide a clear, unbiased view of your facility operations and uncover challenges that must be addressed. No matter your FDA regulated industry, having a formal, thorough and documented audit plan will streamline your audit processes …

Understaffed Restaurants and the Risk of Foodborne Illness
By Jayne Roth, M.P.H, REHS, EAS Independent Consultant At the end of 2021, a survey fielded by the National Restaurant Association, showed that 78% of operators said their restaurant did not have enough employees to support customer demand. Understaffing is not only...
FDA Takes OTC Accelerated Stability Data Very Seriously
Did You Know? FDA Takes OTC Accelerated Stability Data Very Seriously The FDA expects all drug products to bear an expiration date that is backed by scientifically sound data and projections, regardless of whether that drug is an NDA, ANDA or Over the Counter (OTC)...
The 12-Steps of Operational Efficiency
The 12-Steps of Operational Efficiency How Aligning of Operations and Quality Assurance Can Drive Down Costs While Improving Efficiency. Presented by Mike Hughes and Steve Cammarn, Ph.D., EAS Independent Consultants. Operations and Quality Assurance departments...
Drug and Device Corner March 2022
FDA announced the fiscal year 2022 OTC-Monograph Drug User Fee rates in the Federal Register Vol 87, No 51 published today 16 March 2022. These fees cover FDA’s FY2022 which runs from October 2021 through September 2022. The facility fees for FY 2022 are due on 1 June...
Selecting a CRO – an Important Consideration on the Path to Determining GRAS for a Food Ingredient
By Shawna Lemke, EAS Independent Consultant The noble pursuit of ensuring food safety is as old as time. Historians find evidence as far back as ancient Egypt and Rome of dietary laws, labeling and inspections put in place at least in part to address food safety. In...
Cannabis 2.0 – Avoiding Recalls with Shelf-Stability Testing
How cannabis 2.0 product makers can avoid expensive recalls with shelf-stability testing Cannabis 2.0 flooded online stores and retail shelves with exciting new items like infused beverages, chocolates, gummies and topicals. And while there are numerous...
FSPCA Preventive Controls for Human Foods, Part 2 of Blended Course
FSPCA Preventive Controls for Human Foods, Part 2 of Blended Course Presented by Omar Oyarzabal, Ph.D. Senior Consultant. This course is for food manufacturers that need to complete Part 2 of the Blended Class on Preventive Control for Human Food. This online class...
Medical Device Product Risk Management
Medical Device Product Risk Management Presented by John Lincoln, EAS Independent Consultant. Both the U.S. FDA and the EU’s MDR require product risk management as part of virtually all regulatory compliance efforts. Companies must be proactive in reducing...
Navigating the Process of Importing Food Products into the United States
Navigating the Process of Importing Food Products into the United States Presented by EAS, Certified Laboratories and L&L Trade Law. Importers of food products intended for introduction into the U.S. interstate commerce are responsible for ensuring that the...
Susan Braymen
Susan Braymen assists clients with food safety management systems including Food Defense Plans, Food Fraud Prevention, Preventive Controls and HACCP Plans. She performs Food Safety GAP Analysis, cGMP, FSMA, BRC and SQF Audits and creates corrective action plans...
Drug and Device Corner February 2022
FDA’s New Draft Rule: “Medical Devices; Quality System Regulation Amendments” On February 22, 2022, FDA published a proposed rule that aims to harmonize the current Quality System Regulation for Medical Devices under 21 CFR part 820 with international standards, ISO...
Susan Moyers, Ph.D., MPH
EAS Independent Consultant, Susan Moyers, PhD, MPH, is a Food Industry professional with over 20 years of experience in developing, training and auditing food safety, quality, and dietary supplement management systems. She consults with facilities to develop programs...
Are We Even on the Same Team?
Aligning Operations and Quality Drives Down Costs While Improving Efficiency By Mike Hughes, EAS Independent Consultant Have you ever felt like your co-workers are at a different company? Maybe, even a competitor who is trying to run you out of business? Yup – I have....
How To Be an Effective and Sought After Expert Witness
As highly regulated industries are subject to more claims and litigations, and with growing agency challenges to ingredients, processes and branding, the target company’s regulatory compliance is often a key issue in presenting a successful defense. An expert witness’...
The Challenges of Controlling Heavy Metals in Baby Food: Operations, Regulations, & Testing
Food Safety & Quality teams across food industry segments are often tasked with ensuring raw ingredient integrity without impacting production timelines. This balancing act has become even more challenging considering increased product demand, pandemic-related...
Blended Learning – Part of a Culture of Learning
By Nancy Higley, EAS Independent Consultant Continuous learning supports both business and employee goals. When you hire an employee, you are hiring an asset capable of continuous growth. An educated professional can be a point of difference for your business. There...
EAS Consulting Provides Keynote Address at AFIA
EAS consultant Carolyn Kennedy provided the keynote address at the American Feed Industry Association (AFIA) conference during the International Production and Processing Expo (IPPE) in Atlanta. Speaking on human grade pet food, she discussed submissions, labeling,...
Drug and Device Corner January 2022
Reminder: CARES Act Drug Shortage Mitigation Efforts reporting deadlines Reports for calendar year 2020 should be submitted no later than 15 February 2022 Reports for calendar year 2021 should be submitted no later than 16 May 2022 For application holders filing eCTD...
EAS Announces Omar Oyarzabal, Ph.D. as the New Senior Consultant for Food Services
EAS is pleased to announce Omar Oyarzabal, Ph.D. as a new senior consultant for Food Consulting Services. Dr. Oyarzabal, a long-time consultant with EAS, begins this new role today. As a senior consultant, he will work directly with EAS’ Senior Director for Food...
Phyllis Butler-Posy
Phyllis Butler-Posy is a science/engineering regulatory compliance specialist with expertise in technologies for clean drinking water, water reuse and safe processing in the water bottling/dairy/food and beverage industries. She investigates microbiological anomalies...
Roles and Responsibilities for Ensuring the Safety of Animal Food
By Johnny Braddy, DVM, MPH, Dipl. ACVPM, EAS Independent Consultant. (This Issue of the Month article is an abbreviated version of a White Paper published on the EAS website.). To help ensure that the nation’s food supply is safe, Congress amended the Food Drug...
Your Cannabis Business is Only as Strong as the Weakest Link in Your Supply Chain
Product consistency is the most important factor in achieving the holy grail of product manufacturing: return customers. Yet, too many companies are producing inconsistent products due to their lack of insight into their own supply chains. Running the risk of invoking...
Martin Spisak
Martin Spisak assists EAS consumer goods clients with supply chain and quality management issues. He develops, commercializes and facilitates training for new technologies that deliver innovation quality improvements, cost savings and manufacturing efficiencies....
John LoPiccolo
John LoPiccolo is experienced in operations, quality and compliance for the Pharmaceutical, Consumer Products, Medical Device, Food, Supplements and Biologics industries. He helps firms to improve manufacturing operations in quality, cost and delivery functions and...
Roles and Responsibilities for Ensuring the Safety of Animal Food
Prepared by Johnny Braddy, DVM, MPH, DACVPM, EAS Independent...
John Lincoln
John E. Lincoln assists medical device/pharmaceutical companies with production and validation issues including gamma sterilization; QS, 510(k)s, export clearance, injection molding and training. He’s conducted webinars since they first started, workshops and...
Drug and Device Corner December 2021
REMINDER CARES Act Drug Shortage Mitigation Efforts reporting deadlines Reports for calendar year 2020 should be submitted no later than 15 February 2022 Reports for calendar year 2021 should be submitted no later than 16 May 2022 Cover Letter Attachments for...
FDA Issues Draft Guidance on Validation and Verification of Analytical Testing Methods Used for Tobacco Products
On Dec. 21, FDA issued draft guidance for industry entitled “Validation and Verification of Analytical Testing Methods Used for Tobacco Products.” The draft guidance, when finalized, would provide information and recommendations related to the validation...
Thomas Bell Ph.D.
EAS Independent Consultant, Thomas Bell, has a Ph.D. in Microbiology & Physiology from The Ohio State University’s College of Veterinary Medicine & College of Agriculture. His Master’s degree is in Microbiology & Nutrition. He is an expert in quality...
FDA Issues Final Rule for Laboratory Accreditation for Analyses of Foods
The U.S. Food and Drug Administration (FDA) has issued a final rule establishing the Laboratory Accreditation for Analyses of Foods (LAAF) program as required by the FDA Food Safety Modernization Act (FSMA). This LAAF program recognizes accreditation bodies that...
Preparing for TMPs
Are You Ready for TPMPs? Contact EAS for assistance with all of your regulatory needs. Download our quick reference service information sheet or visit our Tobacco services page for more detailed information on how we can help you. Contact Us Tobacco Services...
EAS Senior Director, Tim Lombardo, Is NIE’s Consultant of the Quarter
Tim Lombardo was interviewed in Nutrition Industry Executive as their consultant of the quarter. Tim discusses what makes EAS unique and how the merger of the Certified Laboratories Group of companies and FSNS is a great partnership. Read...
Dairy Processing 101
Dairy Processing 101 A Virtual Training Presented by EAS Consulting Group The FDA’s Food Safety Modernization ACT (FSMA) and Safe Quality Foods 8.0 (SQF) criteria has resulted in increasing compliance requirements to meet stringent safety guidelines, that is...
EAS Welcomes Maged Sharaf, Ph.D. as Senior Director, Labeling, Cannabis and Claims Consulting Services
Alexandria, VA: EAS Consulting Group is pleased to welcome Maged Sharaf, Ph.D. to the management team. As Senior Director, Labeling, Cannabis and Claims Consulting Services, he will be responsible for managing and providing label review and claims substantiation...
Titanium Dioxide – What the EU Ban Means For You
By April Kates, EAS Independent Consultant What is Titanium Dioxide? Titanium dioxide is a substance that has regulatory status in the US for many uses, including as a food additive. In 1966, It was approved to be used in foods at up to 1 percent by weight as a color...
Drug and Device Corner November 2021
Final reminder that we are entering the last month of the registration renewal period for medical device facilities and drug establishments, as well as drug listing certifications. The FDA has provided an Electronic Drug Registration and Listing Instructions website...
Amazon Offers New Documentation Flexibility for Dietary Supplement Selling Partners
Amazon recently announced flexibility for selling partners to demonstrate of Good Manufacturing Practices (GMPs) for dietary supplements. The new policy requires submission of three demonstrations of quality, two of which are mandatory. The third requirement provides...
Mike Hughes
Mike works with EAS clients to improve Operations and Supply Chains. He specializes in improvements in Operational Leadership, Operational Results, Supply Chain Design, Operating Culture, and all aspects of Mergers, Acquisitions, Divestitures & Partnerships. Prior...
Shawna Lemke, Ph.D.
Shawna Lemke has over 20 years of experience contributing to innovation, sustainability and the health of our food system. Occupying a unique position in the science to policy continuum, she manages pre-clinical and clinical phases of drug development, and conducts...
Michael Valentine
Michael Valentine designs, trains, and audits quality systems for manufacturing, customization, and warehousing environments for all FDA regulated businesses. Prior to consulting he was with the Procter and Gamble Company where he led the design, manufacture and...
Nancy Higley, Ph.D.
Nancy Higley has a Ph.D. in Biochemistry from the University of New Hampshire, and Post-Doctorate in Toxicology from the University of Wisconsin. She has conducted applied research in food mutagens and food-borne toxicants at the Food Research Institute at University...Scientifically Sound Specifications No By-Inputs Here
Scientifically Sound Specifications No By-Inputs Here Have you been using “By Input”? FDA deems use of “by input” as unacceptable when exempting a dietary ingredient from a finished product testing. Learn about this misunderstood specification exemption. Purchase this...
Dietary Supplement Good Manufacturing Practices (GMP) for Laboratories
Dietary Supplement Good Manufacturing Practices (GMP) for Laboratories Ensuring Regulatory Compliance Presented by Charlotte Peyton, Independent Consultant EAS is offering an intensive virtual seminar covering FDA’s current Good Manufacturing Practice (GMP)...
EAS Expert Interviewed on The Safety of Imported Ingredients in Food Engineering Magazine
EAS independent consultant, Susan Moyers, Ph.D. was interviewed on regulations and safety of imported food ingredients in Food Engineering Magazine. Learn about FDA and USDA oversight of imported foods as well as particular challenges surrounding standards of...
From Great Nana’s Recipe to Store Shelves: Things to Consider When Scaling Up
Part 1: Labeling Whether it is that recipe that has been handed down from generations, or the one product that is the star of your restaurant or catering business, or perhaps, even something you have spent years perfecting, taking the leap to commercial scale-up can...
Regulatory Considerations for Novel Sports Nutrition Products and Ingredients
By EAS Independent Consultant, David Cockram, PhD Nearly every athlete wants something extra that will provide them with an edge over the competition. Optimizing nutrition is certainly a huge part of improving athletic performance. A well-balanced diet, with...
Drug and Device Corner October 2021
REMINDER that we are in the renewal period for all drug establishment, and medical device facility registrations, as well as the drug listing certification period. The agency announced this month their intention to withdraw the Temporary Guidances for Alcohol-Based...
Top Ten Fatal Flaws in your Food Safety Plan
By Bryan Armentrout, EAS Independent Consultant. “Is your food safety plan ready for a U.S. Food and Drug Administration (FDA) inspection?” Did you know that the average cost of a recall is now over $30 million? That is actual cost; it doesn’t include losses to brand...FDA’s Food Safety Enforcement
FDA’s Food Safety...
Are You Producing Alcohol-Based Hand Sanitizers?
FDA has announced the withdrawal of temporary guidances for alcohol-based hand sanitizers manufactured by non-drug manufacturers during the COVID-19 public health emergency. Effective December 31, 2021, companies manufacturing alcohol-based hand sanitizers under these...
When is a Cosmetic Also a Drug?
Did you know that products designed to clean and beautify that ALSO affect the structure or function of the human body must bear special labeling? Per FDA’s 21 USC 359, these combination cosmetic – OTC Drug products must comply with OTC drug monographs and bear “Drug...
Sunscreen Quality, Safety and Efficacy
New Initiatives by FDA FDA has proposed revisions and updates to OTC-drug sunscreen requirements related to maximum sun protection factor (SPF) values, active ingredients, broad-spectrum requirements, and product labeling, among other provisions. This effort aims to...
FDA Finalizes Two Foundational PMTA Rules
U.S. Food and Drug Administration has issued two final rules for the premarket review of new tobacco products, providing additional information on the requirements for the content, format and review of Premarket Tobacco Product Applications (PMTAs) and Substantial...
Dietary Supplement Virtual GMP Refresher
Dietary Supplement Virtual GMP Refresher The Good Manufacturing Practices (GMP) dictated in FDA’s 21 CFR 111 require that “Each person engaged in manufacturing, packaging, labeling, or holding, or in performing any quality control operations, must have the education,...
Why You Should Outsource Your Food Safety Testing
As a member of the Certified Group and Food Safety Net Services, EAS clients have access to world-renowned testing laboratories that meet your organization’s sophisticated needs. In this column, you’ll hear about their capabilities, environmental challenges and more....
Social Media Considerations for FDA Regulated Products
By Susan Crane, Independent Advisor, OTC Drugs and Labeling Historically, it has been straightforward for the FDA to review and monitor labeling and advertising for products under their jurisdiction, particularly drugs and medical devices. However, the widespread use...
Tim Lombardo Discusses Food Safety for CBD Infused Edibles in NCIA Podcast
Senior Director for Food Consulting Services, Tim Lombardo, recently joined NCIA for a podcast covering food safety of CBD-infused edibles. Listen...
USDA FSIS Revised Guidelines for Labeling Kit Food Products
Those producing nonretail-exempt, multicomponent food kits (such as stir fry and pizza) have a newly revised resource from FSIS: An eight-page booklet with criteria that helps to determine whether the kit product needs to be prepared under FSIS inspection and if so,...
Donald Smith
Mr. Donald Smith is a military veteran who served 29 years in the United States Army. While serving on active duty, as a Veterinary Corps Food Safety Officer, he led many of the Department of Defense’s (DoD) global food safety initiatives. Mr. Smith served four (4)...
Drug and Device Corner September 2021
As part of the 2020 CARES Act, section 506J has been added to the FD&C Act. This section requires manufacturers of certain medical devices to report any interruption or permanent discontinuance in manufacturing to the CDRH. The list of relevant devices can be...
Food Safety – Of the Package By the Package and For the Package
Food Safety – Of the Package By the Package and For the Package Presented by EAS Independent Consultant, Thomas Dunn. Did you know the safety of your product begins and ends with packaging? Packaging is considered the “forgotten food ingredient” and has a...
Drug OTC GMPs and Labeling
Drug OTC GMPs and Labeling A Five-Part Virtual Seminar Series Presented by Lisa El-Shall, Victoria Pankovich, Jeb Hunter New dates to be announced If you manufacture products in the OTC-drug space, including homeopathic, the challenges of FDA regulatory requirements...
Food Labeling Modernization Act of 2021 Introduced
‘The Food Labeling Modernization Act’ (FLMA) was introduced this month in both houses of Congress by Representative Frank Pallone (D-NJ) and Senator Richard Blumenthal (D-CT), along with Representative Rosa DeLauro (D-CT) and Senators Ed Markey (D-MA) and Senator...
What’s Hot in the Regulatory World
By Richard D’Alosio, EAS Independent Consultant I have always described working in regulatory as an iceberg, with the obvious black and white answers to inquiries akin to the visible iceberg floating in the water. However, underneath the water is the unknown or...
Managing Pet Food Ingredients
EAS Senior Director for Food Consulting is featured in an article on pet food ingredients. From safety, to testing to CoAs, the same safety standards apply as with human foods. Read more in Food Engineering Magazine.
Did you know? Color Additives Must Have FDA Approval and (Sometimes) Batch Certification
Color additives for most FDA-regulated products (tobacco and some medical device products are exceptions) must be approved by FDA and listed in the Code of Federal Regulations. If a color additive isn’t listed, its approval must be petitioned and approved by FDA prior...
Environmental Monitoring and Mapping Application (emma®)
Environmental Monitoring and Mapping Application (emma®) By Food Safety Net Services Environmental monitoring programs (EMPs) are critical in food processing environments to ensure the prevention and detection of pathogens. Many of these programs have evolved to...
Do you Make Good, Clean and Healthy Claims on Your Product Labels?
You may be misrepresenting your products by making claims such as good, clean and healthy. FDA has clearly defined definitions for some claims and deciding which to make to market your products can be tricky. Read the EAS authored article in Snack Food and Wholesale...
Drug and Device Corner August 2021
FDA has announced most FY2022 user fee rates this month, conspicuously missing are the OMUFA 2022 user fee rates. We will continue to monitor Federal Register notices and share information as soon as it becomes available. Federal Register Vol. 86, No. 142 announcing...
Is it a Drug or Cosmetic? EAS Podcast hosted by CPhI
As more and more companies operating in the pharmaceutical space are moving into cosmetics, the temptation is to think that life will be much easier from a regulatory and compliance perspective. However, while it is true that pharmaceuticals are more heavily...
Validación de limpieza CIP
Validación de limpieza CIP Presentado por Bernardo Clavijo. Para dar cumplimiento a los requisitos legales y normativos vigentes, es importante realizar la validación de las medidas de control de la inocuidad, entre las cuales está la limpieza y desinfección de las...
What are the Requirements for a Successful DMF Submission for an Active API?
EAS independent consultant, Radhika Rajagopalan answers your questions on DMF submissions in Tablets and Capsules magazine. Under the Generic Drug User Fees Act (GDUFA), the FDA applies a holistic, life-cycle approach to the evaluation of active pharmaceutical...
Find the Right Mix: Flavors and Textures and the Discerning Consumer
By Joe Yun Ph.D. Reprinted with permission from Dairy Foods Magazine. You may have heard the statement, “milk is nature’s perfect food.” Nutritionally speaking, that is true. But is the quality of dairy products we consume always perfect? Have you noticed that when...
Pharmaceutical GMPs, Quality Control, and Data: A Deeper Look at FDA’s FY 2020 FDA Observations
By Amy Scanlin Reprinted with permission from FDLI Update Good Manufacturing Practices—those minimum requirements for methods, facilities, and controls used in manufacturing, processing, and packing of drug products.1 Though clearly articulated by FDA, a review of FY...
Drug and Device Corner July 2021
The FDA has planned a public webinar titled Manufacturing, Supply Chain, and Inspections during the COVID19 Public Health Emergency. Please follow the link to register for this webinar scheduled for 25 August 2021. Recently published in the Federal Register, FDA has...
USDA News: USDA Announces $500 Million for Expanded Meat & Poultry Processing Capacity
USDA has announced a $500 million for expanded meat and poultry processing capacity as part of efforts to increase competition and level the playing field for family farmers and ranchers. This is one of several key steps that USDA intends to take that will increase...
Did You Know? EFSA No Longer Considers Titanium Dioxide E 171 a Safe Food Additive
The European Food Safety Authority (EFSA) no longer considers titanium dioxide E 171 a safe food additive after safety assessments prompted by a request by the European Commission in March 2020. Using Nanotechnology as part of its assessment, the EFSA determined that...
Labeling Risk Management – Five Step Plan
By Ronald J. Levine Food labels are under attack. Every day new lawsuits are filed, often as class actions, as well as through regulatory actions. Legal actions concerning words such as “natural,” or “healthy” are all too common. THE FIVE STAGES OF GRIEF While some of...
CBP and FSVP: Has Your Product Undergone Substantial Transformation?
Are your FDA-regulated food products made with ingredients sourced from outside the U.S.? If so, and if they are further processed, your food product may have resulted in a new or different product that differs substantially from its original state before processing....
FSNS Announcement
We are excited to announce that Certified Group has signed an agreement to merge with Food Safety Net Services (FSNS), a leading provider of laboratory testing services for customers in the food & beverage end-markets. As a result, the newly combined entity will...
Tara Couch Discusses FDA Enforcement of Dietary Supplements in RAPS Regulatory Focus
The pandemic has resulted in an increase in the number of misbranded dietary supplements. As a result, FDA is using updated, alternative, and remote enforcement tools to ensure compliance. Tara Couch, Senior Director for dietary supplement and tobacco services covers...
Cleaning Validations – Your GMPs Depend on It
Cleaning Validations – Your GMPs Depend on It An EAS Premium Webinar Presented by Joe McGuinness, EAS Independent Consultant. For FDA to require that equipment be clean prior to use is nothing new, with the main rationale to prevent contamination or adulteration of...
Did You Know? FDA Amends Standard of Identity for Yogurt
On June 11, 2021, FDA published in the Federal Register a final rule amending the standard of identity for yogurt, 21 CFR 131.200. The final rule, more than a decade in coming, (proposed rule was published in 2009) has been a priority under FDA’s Nutrition Innovation...
Supply Agreements-Defining Acceptable Quality Limits for Packaging
By James Goldman CPP, EAS Independent Consultant “It’s the packaging supplier’s fault!” Package quality disputes between suppliers and customers often start with someone in the filling plant stating “these packages aren’t as good as the ones we ran last week” without...
Meet Our Cosmetics Team
Did you know the EAS Cosmetics Team includes former FDA and high-level industry? From colors to claims, from formulation to safety, our experts can answer any manufacturing and regulatory challenge you may have. Stay in compliance with the FD&C Act. Trust EAS!...
PMTA Deficiency Letter Readiness and Response Preparation
PMTA Deficiency Letter Readiness and Response Preparation Part 2 of a special series focusing on CTP’s requirement of PMTA submissions With Willie J. McKinney, Ph.D., Scientific Advisor for Labstat and EAS Independent Consultant & Moderated by Michael Bond,...
Richard D’Aloisio
Richard D’Aloisio has over forty years in the food industry, with most spent scientific and regulatory affairs functions in multi-billion dollar, multi-national companies including General Foods, PepsiCo, Cadbury (Beverages and Confections), Kraft Foods and Mondelēz...Drug Webinar Registered
Thank you for registering for our webinar! We look forward to having you join us and learning from one of 200 expert consultants. In the meantime, we invite you to learn more about us by downloading our service information sheets. Regulatory assistance is just a click...Dietary Supplement Webinar Registered
Thank you for registering for our webinar! We look forward to having you join us and learning from one of 200 expert consultants. In the meantime, we invite you to learn more about us by downloading our service information sheets. Regulatory assistance is just a click...
June Drug Registration / Listing
As you are probably aware, per 21 CFR 207.57 FDA drug establishment registrants must review and, if necessary, update listing information each June and December. This includes all drug listings, including bulk products. EAS provides support with facility registrations...
Distilling FSMA – Alcohol Beverages and the FDA
Distilling FSMA – Alcohol Beverages and the FDA A complimentary webinar presented by Charles Breen, Senior Advisor FSMA, EAS Consulting Group and John Messinger, Senior Attorney, Lehrman Beverage Law. The question of which federal government agency(s) regulate...
Acidified Foods (AF) & Low-Acid Canned Foods (LACF) Virtual Training
Better Process Control School (BPCS) Training: Acidified and Low-Acid Food Instructed by Omar Oyarzabal, Ph.D. EAS Senior Consultant for Food Services and FDA-Recognized Instructor for BPCS Acidified Foods (AF) Training Low-Acid Canned Foods (LACF) Aseptic Low-Acid...
Foreign Supplier Verification Program Virtual Training
Foreign Supplier Verification Program Virtual Training A Seminar for importers, brokers and distributors of foods, food ingredients and food packaging imported into the United States. FSPCA Developed Curriculum developed by industry, academia and FDA Instructor:...
Do You Have GMP Grief?
EAS pharma expert, Joe McGuinness, published an article in Tablets and Capsules on coming to terms with the requirement of GMP implementation. While GMPs have always been required (in theory) for Active Pharmaceutical Ingredients (APIs), the FDA often found itself at...
Our Consultants are Traveling
and are excited to provide onsite services again! Many EAS consultants are fully vaccinated and are back to traveling to client sites providing that in-person and focused support that sets EAS apart. When you are ready to schedule your next in-person consulting –...
Distilling FSMA – Alcohol Beverages and the FDA
By Charles Breen, EAS Independent Advisor for Food Safety The popularity of new breweries, wineries and craft distilleries has created a community of devoted followers. Many producers do not realize one crucial thing—in addition to the Treasury Department’s Alcohol...
Did You Know? FDA CTP Aims for Two New Product Standard Proposals Within the Year
FDA recently announced a plan to propose product standards banning menthol as a flavor in cigarettes and banning all characterizing flavors (including menthol) in cigars within the next year. If implemented, this ban will apply to all manufacturers, distributors,...
Angie Jacobs
Angie Jacobs is a registered dietitian who enjoys bringing nutrition expertise and regulation conformity together. She has extensive experience in dietary supplement manufacturing, marketing, and development and specializes in dietary supplement label reviews. Angie...
Andy Timperley
Based in the U.K., Andy Timperley works with international client base conducting audits and providing solutions to food and allied industries. He offers practical engineering troubleshooting solutions and methodical approaches to problem solving using principles of...
Daniel Wu
Daniel Wu has a Ph.D. in Food Engineering and joins EAS with 15 years of experience in food safety, QA/QC, research and development in food industry. He is a process authority in thermal processing of LACF and Acidified foods, and an expert in various non-thermal...
Medical Device Manufacturing – A Look at FDA Enforcement Trends
Medical Device Manufacturing – A Look at FDA Enforcement Trends EAS Complimentary Webinar Presented by George Calafactor, Ph.D., EAS Independent Consultant. A look at FY 2020 FDA observations for medical device manufacturers shows a clear focus on procedural integrity...
Drug and Device Corner May 2021
With the Over-The-Counter Monograph User Fee Program (OMUFA) 2021 facility user fee now past due, the FDA would like to remind all drug establishment manufacturers to make payment immediately. Please note the FDA has not issued invoices for this fee. The agency’s only...
EAS Consulting Group Welcomes Tim Lombardo as Senior Director for Food Consulting Services
EAS Consulting Group is pleased to welcome Mr. Tim Lombardo as the new Senior Director for Food Consulting Services. Tim is a widely regarded expert in food safety and microbiology with over 25 years of direct experience leading these programs at a variety of...
Drug and Device Corner April 2021
After regrouping in January, the FDA has published their finalized FY2021 OTC Monograph User Fees in the Federal Register Vol 86, No. 57. This is the official announcement industry has been expecting from the agency which gives the required amount and due date for OTC...
FDA Action Plan for Reducing Exposure to Toxic Elements from Foods for Babies, Young Children
FDA has released an action plan, called Closer to Zero, that aims to reduce exposure to toxic elements in foods intended for babies and young children. This multi-phased approach focuses on levels of arsenic, lead, cadmium and mercury in these foods to the greatest...
Medical Device or a Wellness Device?
Wellness devices are big business, creating an accountability partner for the user and providing real-time data to their medical provider. But where does a wellness device cross the line to a medical device and what does that mean for the manufacturer? From design, to claims to special

PCQI Training: Is it worth it?
by Elise Forward, EAS Independent Consultant I will admit to being biased on the many benefits of becoming your food firm’s Preventive Controls Qualified Individual (PCQI). It’s an important position, one required by FDA for ALL food manufacturing and warehouse...
Fee Rates Under the Over-the-Counter Monograph Drug User Fee Program for Fiscal Year 2021
After regrouping in January, the FDA has published their finalized FY2021 OTC Monograph User Fees in the Federal Register Vol 86, No. 57. This is the official announcement industry has been expecting from the agency which gives the required amount and due date for OTC...
EPCRA SARA SDS RCRA An Alphabet Soup and Customer Notifications Post FDA Regulations
Ingredient suppliers have an alphabet soup of compliance requirements, beyond those of FDA. From EPA to OSHA to even DOT, all product ingredients are regulated depending on the hazards

Flour HACCP Begins with Food Safety Inspections
Consultant Steve Hufford wrote an article on the importance of equipment inspections in support of HACCP efforts, with a discussion of their application specific to flour mills. Read more in Snack Foods and Wholesale Bakery online.
The Human Factor of Regulatory Compliance
Robert Lavieri discussed the human factor of meeting compliance requirements in Food Safety Magazine. Have you asked yourself how best to hold operational, maintenance, quality, and folks on floor, accountable for reliably executing their part of a compliance plan?...
Regulatory Cross Cutting with Artificial Intelligence and Imported Seafood
Angel Suarez shares information on an FDA pilot program that will study and evaluate the utility of AI in support of import targeting, ultimately assisting with the implementation of an AI model to target high-risk seafood products—a critical strategy as the United...
Of the Package, By the Package and For the Package
By Thomas Dunn, EAS Independent Consultant Providing safe food requires both safe food packaging materials and risk prevention by that packaging. As an integral part of the food supply chain, packaging may pose biological, chemical, or physical hazards to the food...
Drug and Device Corner March 2021
Client Updates The FDA has finally taken steps to stop companies that use ‘FDA Certificates’ as marketing tools. The FDA has 2 websites with further information, FDA Calls on Certain Firms to Stop Producing and Issuing Misleading “FDA Registration Certificates” and...
EAS Due Diligence Assessments Support Private Equity and Venture Capital Firms with Acquisitions in FDA and USDA Regulated Industries
In the competitive FDA and USDA space, mergers and acquisitions of brands and companies lead to increased synergies and market share. Without careful and early due diligence assessments, however, these transactions can also increase exposure to regulatory risk. Having...
Medical Foods – When Will the FDA Enforcement “Shoe” Drop?
The industry’s interest in Medical Foods is growing with many new products as well as the repositioning and relabeling of existing products that places them into the medical foods space. FDA’s oversight and enforcement effort focused on Medical Foods has been limited partly …

Elvira Cawthon
Elvira Cawthon works with EAS clients on comprehensive clinical trial protocol development for medical device, IVD and device-drug combination product applications. With a focus on data efficacy, feasibility and usability she also provides ongoing study monitoring...
Clear Regulatory Framework for Hemp and Hemp-derived CBD Products Urged in Bill H.R. 841
EAS is closely monitoring progress of bi-partisan legislation introduced to the 117th Congress on February 4, 2021, H.R. 841, the Hemp and Hemp-derived CBD Consumer Protection and Market Stabilization Act of 2021. The bill seeks a federal regulatory framework for hemp...
Roger Clemens DrPH
EAS independent consultant, Dr. Roger Clemens, is an adjunct Professor of Pharmacology and Pharmaceutical Sciences at USC’s School of Pharmacy as well as Assistant Professor of Regulatory and Quality Sciences. He has also served as a Scientific Advisor for Nestlé USA....
Top Five Considerations for Pre-Submissions
By Elvira Cawthon, EAS Independent Consultant Premarket submissions for medical devices are costly, time-consuming, and denial of a submission can be devastating. Though the FDA Q-Submission (Q-Sub) Program, designed to minimize risk by enabling Sponsors and...
Did You Know? Medical Foods and FDA – Regulatory Scrutiny Ahead
A medical food per FDA is a specific category “formulated to be consumed… under the supervision of a physician and which is intended for the specific dietary management of a disease or condition” which need “distinctive nutritional requirements.” This means that...
Safe Foods for Canadians – What you Need to Know
The Canadian Food Inspection Agency (CFIA) is responsible for the safety of food products sold in Canada by enforcing the recently implemented Safe Foods for Canadians Regulation (SFCR). It is a compilation of 14 sets of regulations governing safety of human …

Drug and Device Corner February 2021
CDER’s Work to Meet User Fee Goals During the Pandemic website includes an overview of the agency’s thinking regarding manufacturing facility inspections during the age of COVID-19. As always, the FDA will use a risk-based approach to identify facilities that...
EAS Senior Director Tara Lin Couch, Ph.D. Featured in NCIA Podcast on the Future of Cannabis Consumerism
EAS Senior Director for Dietary Supplement and Tobacco Services, Tara Lin Couch, Ph.D. was interviewed for the National Cannabis Business Industry’s podcast on the future of cannabis consumerism. Listen now on Apple podcast and Stitcher and hear more about the...
Did You Know? FDA Compliance Includes 21 CFR Part 11 for Electronic Records and Signatures
In addition to Good Manufacturing Practices, supply chain oversight, labeling, registrations, listings, adverse event tracking and all the other regulatory requirements required by FDA, use of electronic records and submissions adds an additional compliance...
USDA Regulatory Requirements for Food Safety
USDA Regulatory Requirements for Food Safety Instructor EAS Independent Consultant Armia Tawadrous, DVM A 12-hour seminar in three-parts, taking place June 14, 16, 18, 2021 From 11am-3pm eastern each day The sensitivity of meat and poultry products to...
USDA Labeling Compliance Virtual Seminar
USDA Labeling Compliance Virtual Seminar Instructor EAS Independent Consultant Susan Glenn If you develop USDA-regulated products you need to understand USDA labeling requirements. USDA labeling has its own set of allowable product names, claims and nutrition labeling...
Carlos Ortiz
Mr. Ortiz is a veteran FDA professional with over 20 years of Agency experience and a veteran of the US Air Force and Army. He served as Regional Activities Manager of the FDA’s Division of Import Operations for approximately ten years. In that role, he was...
Did You Know? Biopesticide Registration is Required by the EPA
Derived from natural materials, biopesticides, are inherently less toxic than conventional pesticides and when used as a component of Integrated Pest Management (IPM) programs, can greatly reduce conventional pesticide use and improve crop yields. EPA has regulatory...
Gisela Leon
EAS Consulting Group’s independent consultant, Gisela Leon, brings in over 33 years of experience in international labeling. She is experienced in USA labeling requirements of food, dietary supplements and cosmetics and in European food laws and multi-language...
Noted Tobacco Expert Joins Labstat’s Scientific and Strategic Advisory Board
EAS sister organization under the Certified Group of companies, Labstat International Inc., has announced that Willie J. McKinney, Ph.D., D.A.B.T., has joined their Scientific and Strategic Advisory Board (SSAB). Dr. McKinney’s regulatory and scientific expertise of...
Cosmetic Regulations, Labeling and Safety Virtual Seminar
Cosmetic Regulations, Labeling and Safety Virtual Seminar How to Ensure Your Products Comply with the US Requirements Instructors EAS Independent Advisor for Colors and Cosmetics, John Bailey, Ph.D. & EAS Independent Consultant, Catherine Bailey Cosmetic products...
Drug and Device Corner January 2021
EAS Client Updates Breaking News: FDA Places All Alcohol-Based Hand Sanitizers from Mexico on Import Alert As part of the U.S. Food and Drug Administration’s continuing efforts to protect consumers from potentially dangerous or subpotent hand sanitizers, the agency...
Preventive Controls for Human Foods Virtual Training with Preventive Controls Qualified Individuals (PCQI) Certification
Preventive Controls for Human Foods Virtual Training with Preventive Controls Qualified Individuals (PCQI) Certification Using the Official Food Safety Preventive Controls Alliance (FSPCA) Training Curriculum Recognized by the US Food & Drug Administration (FDA)...Think You Know Dietary Supplement GMPs?
Think You Know Dietary Supplement GMPs? Take the EAS Challenge Quiz: Not sure of the answers? Master Manufacturing Records must include: A list of all components to be used. A statement of any intentional overage of an ingredient. Instructions for destruction of the...Are your Labels in Compliance with FDA’s Nutrition and Supplement Facts Label Requirements?
Are your Labels in Compliance with FDA’s Nutrition and Supplement Facts Label Requirements? Take the EAS labeling Quiz: A 1 LB pre-cut cake is cut into 10 slices and the RACC for the cake is 80g. What is the serving size? >1 slice (45 g) 2 slices (90 g) 80 g...
Streamline Food Exports to the US
Streamline Food Exports to the US Six Steps to Compliance Success Ensuring compliance with FDA’s Food Safety Modernization Act (FSMA) can be confusing, particularly for foreign-based companies trying to comply with the additional traceability requirements of FSMA’s...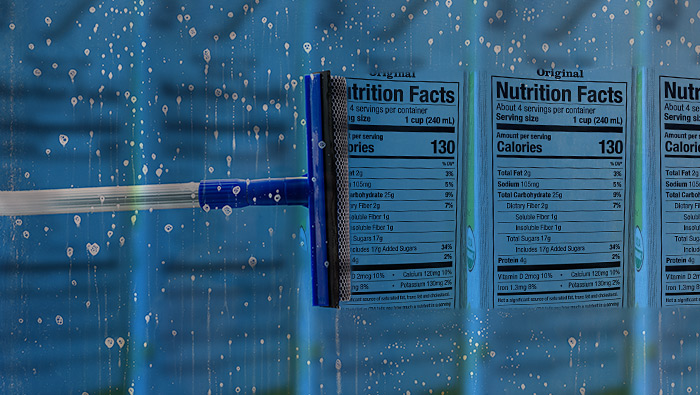
Regulatory Provisions that Can Help Provide Clear, “Clean” and Concise Label Declarations
By Gisela Leon, EAS Independent Consultant and Instructor for Food and Dietary Supplement Labeling Seminar In times of COVID-19, many people are restricted to staying at home, working from home, and cooking at home more than ever before. Cooking at home requires the...
FDA Withdrawal Announcement
The FDA published today in the Federal Register Vol. 86, No. 3 a withdrawal of their December 29, 2020 Federal Register Notice entitled Fee Rates Under the Over-the-Counter Monograph User Fee Program for Fiscal Year 2021./p> With the FDA’s most recent announcement,...
Is your CMO Aware of Their FDA Obligation for OTC-Drug User Fees?
Last week FDA announced the fiscal year 2021 OTC-Drug User Fee rates in a Federal Register Notice. Under the new 2021 rates: Owners of manufacturing and processing facilities of finished OTC-drug dosage forms will pay a full facility fee: $14,060. Owners of a contract...
Did You Know? EAS Assists with Due Diligence Assessments in Support of Mergers and Acquisitions
In the competitive FDA space, mergers and acquisitions of brands and formulations are a common occurrence and help gain a competitive edge. These critical decisions have far reaching implications, including exposure to regulatory risks which can have devastating...
GMPs for Combination Drug-Device Products – Understanding Compliance Requirements for Each Component
EAS published an article in MedTech Intelligence on the importance of component GMP requirements for drug-device combination products. “The rapid development of combination products—biologics, pharmaceuticals and their devices—provides great opportunity for...
Victor Manuel Fernandez Rivera
Victor Fernandez Rivera utilizes extensive experience as a biotechnology engineer to facilitate compliance with food safety systems pertaining to FDA and USDA regulations for EAS clients. He leads projects aligned with GFSI standards as well as HACCP, FSMA and FSIS...
Wishing You a Healthy and Happy 2021
From our family to yours, EAS Consulting Group wishes everyone a healthy and happy New Year! 2020 will certainly be remembered as one of immense challenges and creative opportunities. From the push to develop effective testing and vaccines, to supply chain issues that...
Recall Plans and Strategies
Robert Fish, Independent Advisor, Quality and Compliance Every year FDA monitors thousands of recalls of regulated products (over 7,000/year). Though most product recalls are voluntary, the FDA can also order them. Recalls can be very damaging to the reputation of a...
FDA OTC-Drug User Fee Rates Established for FY 2021
FDA announced the fiscal year 2021 OTC-Drug User Fee rates in a Federal Register Notice published today, 12/29/2020. The notice covers all qualifying manufacturers and processors of finished dosage form OTC monograph drugs, including contract manufacturing facilities...
Did you Know? 3 Key Considerations for Developers of Wearable Software as Medical Devices
Digital platforms for wearables can serve both medical and non-medical purposes. But, when software technology helps the wearer or medical professionals identify and track information in support the diagnosis or treatment of a disease, that software is considered a...
New USDA FSIS Requirements for Shell Eggs – Top 5 Things You Need to Know Now!
USDA Food Safety Inspection Services (FSIS) recently issued the first regulatory update of the Egg Products Inspection Act (EPIA) since 1970, called the Egg Products Inspection Regulations (EPIR) which modernizes the industry requirements and FSIS inspection of shell...
The Cosmetic-Drug Conundrum
The subtle differences between allowable cosmetics claims and those crossing the line into drug products is tricky. The wrong claims can cause a cosmetic product to be misbranded attracting unwanted FDA attention. Once some of your marketing claims are under review,...
Micro Quality Labs Joins the Certified Group Family
We are proud and excited to announce that Micro Quality Labs (MQL) has joined the Certified Laboratory family of companies. MQL is an ISO accredited, independent analytical testing laboratory, providing state-of-the-art instrumental chemical and microbiological...
US FDA Proposed Traceability Rule – Overview
FDA’s proposed rule “Requirements for Additional Traceability Records for Certain Foods” mandates traceability recordkeeping requirements for certain foods such as cheeses, shell eggs, some types of fish and produce. When finalized, the Traceability Regulation will be …

Drug and Device Corner November 2020
Revisions to section 801(e)(4)(E)(iii) of the FD&C Act as part of the Coronavirus Aid, Relief, and Economic Security (CARES) Act direct FDA to provide certification for Devices Not Exported from the U.S. Manufacturers of devices not exported from the United...
EAS Celebrates One-Year as a Member of the Certified Group of Companies
November marked the one-year anniversary for EAS Consulting Group’s acquisition by Certified Laboratories as part of the Certified Group of companies – and what a great year it has been! While both companies knew the synergistic capabilities of EAS regulatory...
Genome-Edited Crops and the National Bioengineered Food Disclosure Standard: Can Regulators Find the Right Balance?
Anyone who follows science news has probably heard of the phenomenal scientific advancements using CRISPR. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) exist in nature as a defense mechanism in bacteria against invading viruses. In 2012, researchers Jennifer Doudna and

Food Safety and Artificial Intelligence Published in IAFP Food Protection Trends
Members of IAFP are invited to read an article on Food Safety and Artificial Intelligence written by Independent Consultant, Mehrdad Tajkarimi, Ph.D., and published in IAFP’s Food Protection Trends. Today, the food industry has more data than capacity for analyzing...
Joseph McGuinness
EAS consultant Joe McGuinness utilizes extensive experience in the pharmaceutical industry to assist EAS clients with preparation for and execution of both internal and FDA audits. He designs protocols for SOPs, GMPs and training programs ensuring an understanding of...
Allen Sayler Awarded International Dairy Federation Award of Excellence
Senior Director Allen Sayler has received the Award of Excellence by the Brussels-based IDF for outstanding contributions to their programs. Allen is an internationally recognized expert in dairy and dairy process, having previously worked as the Vice President of...
Did You Know? Environmental Monitoring and Testing of Pathogens is a Critical Must for Ensuring FSMA Compliance
The emphasis on proactive management of issues that could cause a food safety hazard, per FDA’s Food Safety Modernization Act, requires firms to improve controls for a variety of issues at all levels. One hot-button concern that continues to wreak havoc in the...
Dairy Microbiology and Spoilage Article Published in Dairy Foods Magazine
EAS consultant Sarah Goreham published an article in Dairy Foods Magazine on rethinking microbiology and safety protocols to prevent dairy spoilage with particular examples given for Bacillus cereus. Prevention is, as always, is our best defense to minimize our risk...
Peter Poteres
Peter Poteres provides consulting services for all aspects of Food Safety and Quality. He specializes in trouble shooting, organizational structure and food safety/quality preventative programs utilizing over 40 years of hands on experience in Quality Assurance,...
Allen Sayer was Awarded Honorary Life Member by the IAFP
Allen Sayer was awarded Honorary Life Member by the International Association of Food Protection (IAFP) at their annual meeting. This award, through nomination by industry colleagues, recognizes Allen’s career achievements and contributions to food safety. You may...
Preparing for a PMTA Pre-Approval Inspection
Presented by Tara Lin Couch, Ph.D., EAS Senior Director for Dietary Supplement and Tobacco Services The Family Smoking Prevention and Tobacco Control Act referred to as the Tobacco Control Act (TCA), signed into law on June 22 …

New Ingredients in Pet Food Formulation: Not as easy as creating a product with a few trending ingredients
By Carolyn Kennedy Everyone is looking for the next best thing when it comes to pet food. Many companies have tried to introduce new ingredients. Often, the new ingredients in pet foods mirror the trending ingredients in human food. There has been an influx of CBD and...
Did You Know? Equipment Sanitation and Dietary Supplements – Which cGMPs Apply to Me?
As a dietary supplement firm, and a subcategory of foods, how do you decide which cGMPs to follow? The answer is determined by what specifically you produce – dietary supplements or dietary ingredients. Once that is understood, the cGMP operational requirements,...
GMPs for Hemp and CBD?
Developing solid GMP protocols is essential for firms operating in the hemp and CBD space. Read EAS independent consultant Charlotte Peyton’s blog published on the National Cannabis Business Industry (NCIA)’s website. Join EAS for our upcoming EAS short course on...
Drug and Device Corner September 2020
EAS would like to remind all eNews readers of the importance of an appointed U.S. Agent. The FDA recently deactivated the FDA registrations of 340 foreign establishments that failed to identify a U.S. Agent as required by FDA’s regulations. As we head into the FDA...
EAS Becomes GRMA Trusted Partner
The Global Retailer and Manufacturer Alliance (GRMA) recently confirmed EAS as a Trusted Consulting Partner for their member organizations. As a Trusted Partner EAS assists GRMA member suppliers and vendors with regulatory operational support. Acceptance into the...
House Vote on Legalization of Marijuana Delayed
Of interest to the hemp, CBD and cannabis industries, the US House Democratic leaders have decided to postpone voting on legislation to decriminalize marijuana at the federal level until after the November 3, 2020 election. EAS team of consultants, under leadership of...
Elise Forward
Elise Forward provides food safety oversight for processes, products and raw materials including risk assessments. She assesses supplier programs to ensure compliance with regulatory, customer, and internal requirements and quality systems. She also designs,...
Food Legislation in the EU – An Introduction
Food Legislation in the EU – An Introduction An EAS Short Course Presented by Michael Hickey, EAS Independent Consultant If you sell food products in the EU, understanding EU Food legislation is critical to success. Join EAS Consulting Group’s expert in EU Food...
California Proposition 65 and the Food Industry
Proposition 65 is a statute that all companies selling products into California must comply with by providing warnings if their products contain certain chemicals that result in consumer exposure above a certain daily threshold. Failure to comply with the stringent requirements …

Process Validation for OTC Liquids and Topical Products
Process Validation for OTC Liquids and Topical Products Presented by EAS Independent Consultant, Miguel Montalvo If you manufacture OTC liquids, such as hand sanitizers, and other topical products, an effective Process Validation Program is an FDA requirement. Do you...
Developing Quality Systems for the CBD and Hemp Industries – EAS Short Course
As states begin to regulate legalized CBD and hemp, the concern of how Good Manufacturing Practices (GMPs) apply to these unique industries cannot be understated. While regulations vary from state to state the quality systems under which products are grown …
Hygiene Improvement, Monitoring & Tracking
While the “new norm” for food manufacturing hygienic practices is rapidly evolving, an intense focus on proactive assurance of food and employee safety is the top industry priority. With increased focus on hygienic practices comes an increased requirement to data …

Understanding and Mitigating Risks of Emerging Pathogens
The food industry has a history of testing for Enterobacteriaceae, coliforms and/or E. coli as indicator microorganisms. Since 2009 there were 40 outbreaks in the United States of pathogenic E. coli such as Shiga toxin-producing E. coli (STEC) associated with leafy greens…

Did you Know? EAS Assists with California Prop 65 Requirements
Proposition 65 requires specific warnings be applied to products sold in California that contain any one of the ~900 chemicals on the Proposition 65 list, whereby use of the product results in consumer exposure to those chemicals above Safe Harbor Levels. EAS is a...
Sarah Goreham
Sarah Goreham is a food manufacturing consultant whose has worked with billion-dollar companies to start-ups across the country. She develops long-term process improvements and best practices to keep consumers safe and our industry profitable and assists with...
Daryl Pilmore
Daryl Pilmore has provided expert regulatory and technical advice for companies with products going into markets in Australia and New Zealand for over 20 years. He works with cosmetics, personal hygiene and domestic cleaning products (including hand sanitizers and...
Michael Willard, Ph.D.
Dr. Willard is a graduate of the Texas A&M University, College of Veterinary Medicine. He is a Professor Emeritus of Small Animal Clinical Sciences and specializes in gastroenterology, hepatology, pancreatology and endoscopy. Dr. Willard has held faculty...
Jayne Roth
Jayne Roth is a Food Safety Advisor with a master’s degree in public health. Her special interests include infectious disease control and food safety rule interpretations. She is well-versed in food safety regulatory compliance and regulatory structure of public...
Sivan Ananth
Sivan Ananth has 28 years of experience in the Aquaculture and seafood industries and 20 in the pasteurized crab meat industry. He has a B.S. in Fisheries Science and an M.S. in Coastal Aquaculture. Sivan is skilled in the Food Safety Management Systems of BRC, HACCP,...
Maintaining Compliance through Virtual Audits
When the phone rings at EAS Consulting Group lately it is very often someone seeking assistance with Good Manufacturing Practices. While COVID-19 has caused major disruptions to global supply chains and manufacturing capabilities, FDA regulated industries forged ahead…

Using Legal Dietary Ingredients in Sports Nutrition Products
James Hoadley, Ph.D. wrote an article on ensuring use of legal dietary supplement ingredients for Natural Product Insider. Regulators have a fine focus on dietary supplement products that are mislabeled or misbranded, and brands must be careful when it comes to...
Greg Bikofsky
Greg Bikofsky provides food safety consulting and program development services to food manufacturers, retail food and the food service industries. His capabilities include SQF and FSSC22000 program development and audit preparation, food safety plan development,...
Cleaning-In-Place (CIP) Validation
“Validation” is a term and concept that is widely used but is also widely misused and misunderstood. Validation of food safety control measures is currently a mandatory requirement in US, other countries and in internationally accepted regulations, but also in GFSI certification schemes, i.e. SQF, BRC, FSSC22000 and IFS. Several FDA and USDA regulations including FSMA Preventive Controls, LACF/AF…

Did You Know? EAS Offers USDA Consulting Services, Including Labeling Assistance and Organic Requirements
EAS independent consultants include experts in USDA regulations. From the unique requirements of USDA labeling to food safety of domesticated meat products and shell egg products, you can be confident that EAS offers accurate and thorough advice and actionable...
EAS Authors Chapter in Present Knowledge in Nutrition 11th Edition Published by Elsevier
Just released on July 31, 2020, EAS Independent Consultants Betty Campbell, James Hoadley, Ph.D. and Robert Post, Ph.D. co-authored an article on Nutrition in Labeling including topics such as mandatory and optional nutrition label information in specified formats and...
How to Perform an OOS Investigation for Tablets and Capsules magazine
Senior Director for Dietary Supplements and Tobacco Services, Tara Lin Couch, Ph.D. authored an article on How to Perform an OOS Investigation for Tablets and Capsules magazine. EAS is a recognized industry leader for regulatory services to the dietary supplement...
Siva Hari, Ph.D.
Siva Hari, Ph.D. assists dietary supplement, pharmaceutical and device clients with revitalization of QC and FDA-compliant regulatory operations. His expertise includes strategic planning and project development, product design and R&D. He facilitate QA and QC...
Hygienic Equipment Sanitation – Best Practices for Food Safety
This short course offers practical information on best practices, “real-world” examples and tips on how to enhance daily operations related to improving existing food safety and quality programs. The three-session training will focus on criteria for selecting, installing and maintaining processing …

Did You Know? EAS is now aligned with Certified Laboratories and its subsidiaries, Labs-Mart, LabStat and ABC Testing
As you read in the December 2019 edition of EASeNews, EAS is now a member of the Certified family of companies. This means clients can enjoy additional regulatory benefits of product testing and validation through our partner organizations in the U.S. and Canada. From...
Enhancing Food Safety Through Technology
If you missed EAS independent consultant Dr. Susan’s Moyer’s presentation for a Food Safety Strategies webinar on food technology and food safety, it is now available on-demand on the Food Safety Strategies website. EAS offers comprehensive solutions …

Bernardo Clavijo
Bernardo Clavijo is a food safety expert with expertise in regulatory requirements of Codex Alimentarius and standards requirements of GFSI certification schemes. He assists EAS clients with validation of thermal processes, the creation of technical documentation in...
Aisha Qadeer Siddiqui
Aisha Siddiqui is a Bio-Medical Engineer with exceptional analytical abilities and goal-directed thinking. She has over 10 years of diverse regulatory experience in manufacturing and contract manufacturing environments. Aisha has Quality Control/Quality Assurance...
Cell-Cultured Meat Products and Plant-Based Protein Analogues
An EAS Consulting Group White Paper discusses regulatory aspects and consumer trends in cell-cultured meat products and plant-based protein analogues.
As consumer acceptance …

Selecting and Submitting an ANDA Application
The FDA Generic Drug User Fees Amendment (GDUFA) has entered a relatively matured stage with applicants of Abbreviated New Drug Applications (ANDAs) experiencing a timely review and communications regarding their submissions packet, as well as increasing approval numbers. However, until …

Retail Food Safety in a Post-Pandemic World
The Retail Food Industry is finally beginning to see an opening as many areas of the country are experiencing decreasing cases of COVID-19, meaning businesses can begin to safely scale up. But what does that mean and how can the retail food industry ensure safe operations in this new normal? Do you understand …

Did You Know? EAS Helps Firms Prepare for FDA Inspections Against Intentional Adulteration Rule
FDA began inspections for Intentional Adulteration (IA) Rule components in March 2020, including assessment of Food Defense mitigation strategies, verification of effectiveness and the development of corrective action steps to be taken in the event that the mitigation...
Brad Douglass, Ph.D.
Brad Douglass evaluates FDA and FTC compliance of dietary supplement materials including review and audit of dietary supplement labels and labeling. He is experienced in multiple technical, quality, and formulation roles in the dietary supplement and cannabis...
April Kates
April Kates has been an EAS Independent Consultant since 2017. In the time she has worked with EAS she has provided interpretation of FDA food labeling rules and reviewed for regulatory compliance food and cosmetic labels and labeling. In addition, she has provided...
Personal Care and Healthy Aging
As the overall population of the U.S. continues to gain longevity, consumers demand personal care products that address the signs and symptoms of aging. Everyone gets older, but no one wants to feel or look older.
Consumers are willing to pay a …

Good Manufacturing Practices – Comprehensive Equipment and Utility Change Control for GMP Production Facilities
Many companies limit change control to documentation, such as batch records, SOPs, protocols and specifications, while handling equipment and change control in isolation. As a result, new equipment is often installed and connected to utilities without input from facilities, engineering, validation and …

Biosimilar Biological Products: Development & Applications
The US Food and Drug Administration (FDA) is responsible for advancing the public health by helping to speed innovations that make medicines safer and more effective and by helping the public get the accurate, science-based information it needs to use medicines to...
Dietary Supplement Contract Manufacturing Partnerships and Regulatory Compliance
For a number of years, many dietary supplements have been produced by contract manufacturers. As manufacturing processes become more sophisticated (e.g., complex probiotics) and FDA regulatory requirements continue to expand, it becomes more cost-effective to use the...
Lisa Zitiello
Lisa Zitiello has over 25 years in professional evaluation, teaching and practical application of safe food handling techniques. Her expertise is seafood processing and HACCP program development, GFSI and GMP audit preparation, FSMA Preventive Controls for Human Food...Allen Sayler Receives IAFP Honorary Life Member Award
EAS is pleased to announce that Senior Director for Food Consulting Services, Allen Sayler, has been awarded the prestigious International Association of Food Protection (IAFP) Honorary Life Member. This award, through nomination by industry colleagues, recognizes...
Reality Due to Globalization
The year 2018 witnessed the 100th anniversary of the influenza H1N1 pandemic, sometimes referred to as the 1918 Spanish Flu. Despite being coined the Spanish Flu, its origins are unknown. That pandemic, which killed an estimated 50 million worldwide and 675,000 in …

Writing Effective SOPs Can Influence Compliance
EAS independent consultant, Heidi Stuttz discussed how writing effective SOPs can influence compliance and build a better organization in Drug Development Delivery. Drug firms must devote time, diligence, and meticulousness in the development of safe product design, materials …

New On-Demand Webinar – OSHA Employee Safety Requirements of Lockout-Tagout
If you missed the EAS webinar covering the critical steps to the life-saving Lockout-Tagout, the complimentary webinar is now available on-demand on the EAS website. Understand the steps to safely disable equipment in need of repair and maintenance as well as OSHA’s...
Did you know? EAS Offers Submission Support for CDER eCTD Applications
Did you know there are exemptions/waivers for FDA’s eCTD requirements for CDER applications? In February 2020, the FDA released a Guidance Document regarding eCTD requirements for regulatory submissions. This guidance document describes how content must be organized...
Unique Device Identifiers – Are Your Products in Compliance?
EAS reminds all medical device firms that the compliance date by which devices must have a UDI is approaching. For more details on this requirement we invite you to read an EAS authored article in MedTech Intelligence. Timely compliance will ensure that your products...
Sensory Considerations for Reformulating Food Products with Reduced Sugar and Sodium
Former EAS Consulting Group Independent Consultant, Rebecca Harter, discussed sodium and sugar reduction considerations in an article published in Natural Products Insider. Whether reduction is achieved by direct replacement, lowering formulation levels or the...
Active Managerial Controls for Food Safety – On-Demand Webinar
EAS independent consultant and former CDC Environmental Health Officer / Deputy Chief, Charles Otto, presented a webinar for the National Restaurant Association on Active Managerial Controls for Food Safety. AMCs are a great enhancement to Food Code-compliance...
Kristi Smedley, Ph.D.
Kristi Smedley assists clients with submissions such as GRAS, animal and human food additives, feed ingredients and dietary supplements. Her specialties include study development and conduct of animal research. She is a former Chief, Petitions and Regulations Staff,...
Carolyn Kennedy
EAS Consulting Group’s Carolyn Kennedy works with pet food companies on new and innovative product development and formulae, helping them to meet market needs and demands. As an independent consultant, she assists with brand management and nutritional support,...Fast Tracking Antimicrobial Agents – FDA’s Accelerated Programs
Developers of antimicrobial agents may seek an accelerated FDA review of their products under the accelerated programs such as Fast Track Designation, Breakthrough Therapy Designation, Accelerated Approval, or Priority Review and gain additional 5 years of …

OSHA Lockout-Tagout – Critical Steps to Employee Safety
Is your manufacturing floor a safe work environment? Do you abide by OSHA controls commonly known as Lockout-Tagout? Employees can be seriously or fatally injured when machinery they clean, service or maintain unexpectedly energizes, starts up, or releases …

James Hoadley
During Dr. Hoadley’s 20-year FDA career, he participated in the development of NLEA-implementing nutrition labeling and health claim regulations. As a Senior Regulatory Scientist in the Office of Nutritional Products, Labeling and Dietary Supplements (ONPLDS) Dr....
Building Food Product Development Branding and Messaging
Food companies are striving to be a force for good and today many are developing products to accelerate wellness. That’s because consumers are prioritizing health consciousness as part of their everyday food choices, according to the IFIC Foundation 2019 Food and Health Survey. Price, taste and convenience are no longer the reigning factors. And, for many consumers, a food label alone is not sufficient to convey the information they seek to make a choice. Consumers are weighing a new set of factors and choosing food and beverage products that promote their health and wellness. To that end, food manufacturers must ensure that their business functions and product messaging convey integrity of brand, promotes transparency, and are messaged beyond the label to be appealing.
Sayler and Couch Discuss Importing Challenges for Dietary Supplements Under FSMA and FSVP
EAS Senior Directors Allen Sayler and Tara Couch, Ph.D. discussed importing challenges of dietary supplements, particularly in light of the Foreign Supplier Verification Program in Food Safety Magazine. Dietary supplement firms must comply with GMPs set forth in 21...
James Goldman
James Goldman is a certified packaging expert focusing on package development, supply chain, and production equipment and has deep knowledge of design, manufacture and distribution and supply chains. Goldman is a glass container expert applying knowledge to reduction...
Sousan Sheldon, Ph.D.
Sousan S. Sheldon, Ph.D. is a former FDA Primary Reviewer, Supervisory Reviewer, Scientific Policy Advisor, and International Policy Analyst in the FDA’s Commissioners’ Office for medical devices and pharmaceuticals with expertise in various FDA Centers...Independent Consultant Angel Suarez Covers Food Code Guidelines
Angel Suarez was interviewed on GMPs and Food Code 2017 Guidelines in an article published in Food Safety Strategies. In the food area, the GMPs ensure that ingredients, products and packaging materials are prepared, presented and handled safely and that food products...
Paula Trumbo, Ph.D.
Paula Trumbo works with clients on food and dietary supplement labeling, claims, and other nutrition related issues for compliance with FDA regulations. Prior to consulting, she led FDA’s Nutrition Science Review Team responsible for the pre-market review of the...
Cutting Edge Methods for Detecting Food Fraud
The challenge of detecting Food Fraud has never been greater nor the economic loss to food manufacturers, importers, retailers and consumers. In the recent past, we have had to rely on the integrity of the supply chain, person to person relationships and trained sensory
Therapeutic Nutrition: Potential and Challenges for Novel New Products
David Cockram, Independent Consultant, EAS Consulting Group, LLC We live in exciting times, with new discoveries being announced daily about the relationships between nutrition, diet, health, and disease. So much so that it’s often hard to keep track of whether this...
Did you Know? Requirements for Animal Food/Feed Manufacturers Include Preventive Controls
Preventive Controls for Animal Food/Feed is a requirement for all manufacturers of animal food/feeds. EAS expert, Jerry Poley, who assists clients with food safety audits including requirements for HACCP, FAMI QS, FPA Safe, PAACO (Professional Animal Auditor...
Tips to Streamlining the Drug Master File Process
The meticulous detail of a Type II Drug Master Files (DMF) enables FDA to review and assess the chemistry, manufacturing, stability, purity, impurity profile, packaging and Good Manufacturing Practices data of Active Pharmaceutical Ingredients (API) or a finished drug dosage form …

Bryan Armentrout
Bryan Armentrout is an expert in dairy, quality, system development, Safe Quality Foods, (SQF), Hazard Analysis and Critical Control Points (HACCP), design control, auditing, troubleshooting, recall, crisis management, and process improvement. Prior to consulting he...Greg Weilersbacher on Change Control for GMP Production Facilities
Many companies limit change control to documentation while handling equipment and facilities in isolation, but that practice can wreak havoc on GMP operations.
Compliance with Mobile Medical Devices Discussed in MedTech Intelligence
Device companies seeking to develop products that include a mobile app must ensure regulatory compliance with product and data safety.
Top Five Hurdles for FDA ANDA Submissions and Approvals
By Radhika Rajagopalan The FDA Generic Drug User Fees Amendment (GDUFA) has entered a relatively matured stage with applicants of Abbreviated New Drug Applications (ANDAs) experiencing a timely review and communications regarding their submissions packet, as well as...
Garth Kahl
Garth Kahl has a long career history working with organic crop and livestock farms and processing throughout the U.S. and Latin America to ensure compliance under NOP, EC 834/2007, Mexico LPO, COR and JAS standards. He assists EAS clients with all aspects of organic...Couch Authors Article on GLPs for Dietary Supplement Contract Labs
Supplement brands that partner with contract labs must ensure they are following good lab practices and other quality assurance programs.

Did You Know? The FDA Uses DUNS Numbers to Verify Company Information!
Data Universal Number Systems, commonly known as DUNS are unique, site specific, nine-digit identification numbers provided by Dun & Bradstreet (D&B) and used globally. Free to obtain, FDA and other federal agencies use DUNS numbers to track and verify company...January 2020 Drug and Device Corner
Is your labeler code up to date?!? Chances are if you have not thought about this file since you originally requested a labeler code from the FDA, it could use an update. We saw during the drug listing certification period, that several labeler codes were reflecting...Couch Co-Authors Article in FDLI Update on PMTAs for Deemed Products
In October the FDA Center for Tobacco Products (CTP) held a Public Meeting about Preparing Marketing Applications for Deemed Products. A summary of that meeting was co-prepared by Tara Lin Couch, Ph.D. and published FDLI Update magazine.
Steve Armstrong Interviewed on Marketing Claims
Consumers demand certain qualities—think clean label, zero sugar and non-GMO, for example, and marketing claims can affect the ways that foods and beverages are produced.
Comprehensive Equipment Utility Change Control GMP Production Facilities
Tablets and Capsules January 22, 2020 Comprehensive equipment and utility change control for GMP production facilities Greg...How Food and Beverage Marketing Claims Can Affect the Production Process
Food Engineering January 17, 2020 https://www.foodengineeringmag.com/articles/98672-how-food-and-beverage-marketing-claims-can-affect-the-production-process Steve Armstrong
Codex Opportunities for Food Manufacturers
Can Codex food standards and various food safety, hygiene guidelines and codes of practice positively impact domestic food manufacturers that do not export? The short answer is “YES!” Codex provides a transparent international platform for food safety and hygiene as well as manufacturing practices, food …

Marc Ullman
Of Counsel at Rivkin Radler, LLP. Attorney Marc Ullman represents clients in matters relating to all aspects of the firm’s practice, including Food and Drug Administration and Drug Enforcement Administration matters, regulatory issues, Federal Trade Commission...Developing a Mobile Medical Device? FDA Is Watching
MedTech Intelligence January 6, 2020 Developing a Mobile Medical Device? FDA Is Watching Amy...
David Cockram, Ph.D.
David Cockram assists EAS clients in the areas of GRAS, Infant Formula and NDI study design, execution and monitoring in support of safety submissions for novel food and dietary ingredients and nutritional products. He recently retired as Senior Director of Global...
Radhika Rajagopalan
Radhika Rajagopalan, Ph.D. is a former Quality Assessment Lead, Expert Reviewer in ANDA Stability Testing at FDA. She has decades of experience with CMC packages including ANDAs, DMFs (Type 2 and 4), INDs, Bio-INDs, OTCs, supplemental new drug applications, novel...
Jon Anderson
Jon Anderson assists EAS clients with occupational safety standards and compliance, conducting audits and customized trainings for manufacturing plants, distribution centers and warehouses to ensure a culture of safety specific to each work environment. He provides...When you Need an Expert – Ask EAS!
In this month’s Ask the Expert column, we thought we’d start this new year by taking the opportunity to humbly share why we at EAS are confident that our staff and consultants offer the best expert regulatory knowledge in the industry, providing proactive and accurate...FDA Cites Multiple Violations for Selling CBD as Supplement, Food, Cosmetic or Animal Food Ingredient
By Gisela Leon, MS, MBA, Independent EAS Consultant On November 22, FDA published 15 warning letters in a “catch all” effort regarding cannabidiol products. The products range from articles sold as dietary supplements, conventional foods, cosmetics, and animal...December 2019 Drug and Device Corner
Guidance Document updates on the FDA website All divisions Certificates of Confidentiality (distributed for comment purposes only) – This draft guidance describes FDA implementation of the revised provisions applicable to the request for, and issuance of, a...Dietary Supplement and FSMA EAS Webinar – On-Demand
Did you know that some parts of FSMA also apply to dietary supplements? Watch our EAS on-demand webinar to ensure your understanding with requirements.
FDA’s CTP Updates Industry on Premarket Tobacco Product Applications for Deemed Products
If you missed FDA’s public meeting on PTMAs, click here for a summary co-prepared by EAS and published in the Food Drug Law Institute Update magazine.

Julie Litz
Julie Litz’s career as a quality director has covered Food, Dietary supplement and Animal Feed industries. She has extensive experience in setting up food safety plans for manufacturing facilities and transitioning a facility from food regulation compliance (CFR 117)...Best Practices in Equipment Sanitation – An EAS On-line Short Course starting January 29, 2020
Equipment sanitation is at the heart of food safety and quality programs. From selecting, installing and maintaining processing equipment to documentation of FSMA compliance, these “best practices” are critical.
Contract Labs for Safe, Compliant Supplements
Natural Products Insider December 6, 2019 https://www.naturalproductsinsider.com/labstesting/contract-labs-safe-compliant-supplements Tara Lin Couch, Ph.D.
Dietary Supplements and FSMA Compliance – Fallacy or Fact?
In 1994 the Dietary Supplement Health and Education Act (DSHEA) created a new, legal class of products, called “Dietary Supplements”, which are regulated by the FDA as a subcategory of foods. Since DHSEA, the Food Safety Modernization Act (FSMA) of 2011 was passed
Safety Data Sheets – A Requirement for Safe Manufacturing Operations
Food Quality and Safety Magazine published an article written by EAS Independent Consultant Robert Kapp Safety Data Sheets (SDS) covering their importance and utilization in manufacturing operations. SDS, are a critical component, required by law, containing all...Ready to be an Expert Witness? Concerned about Dietary Supplement GMPs?
If you missed our recent webinars you are now able to view them free on-demand on the EAS website. Our Expert Witness bootcamp, presented by EAS Independent Consultant and General Counsel at Herrick Feinstein, Ronald Levine, and his colleague at Herrick, Leah Kelman,...Are You Making Natural Color Claims On Cosmetic Products?
FDA has stated all color additives are synthetic, so “natural colors” in cosmetic products must be an inherent color of an ingredient, not added for coloring, says John and Catherine Bailey, EAS Independent Consultants and experts on cosmetics. Their article on...Your Food Additives May Need GRAS
As innovative food companies develop an array of products to satisfy a discerning consumer, the question of whether food additives intended for use in those products are safe for their intended uses. According to FDA, “GRAS” is an acronym for the...November 2019 Drug and Device Corner
REMINDER: we are at the end of the renewal period (1 Oct – 31 Dec) for medical device & drug establishment registrations, as well as drug listing certifications. Please factor holiday schedules as well as the potential of a U.S. government shutdown into your...FDA Holds Public Meeting on PMTAs for Deemed Products Meeting Summary
FDLI Update November 26, 2019 https://www.fdli.org/2019/11/fda-holds-public-meeting-on-pmtas-for-deemed-products-meeting-summary/ Tara Lin CouchHitting FSMA Benchmarks in Warehousing and Distribution Networks
Food Safety Strategies November 15, 2019 https://www.foodsafetystrategies.com/articles/1049-hitting-fsma-benchmarks-in-warehousing-and-distribution-networks Purnendu Vasavada, Penny Vyskocil
Dietary Supplement GMP Enforcement – a Look at Recent FDA Observations and Warning Letters
Though FDA’s 21 CFR 111 GMPs for Dietary Supplements has been in place for over a decade, the industry continues to be plagued by the complexities of compliance…
October 2019 Drug and Device Corner
REMINDER: we are currently in the renewal period (1 Oct – 31 Dec) for medical device & drug establishment registrations, as well as drug listing certifications. The FDA, in their commitment to assisting industry in the development of affordable, available, generic...Navigating CBP and Prior Notice of FDA’s Imported Products
As the pace of FDA regulated products increases, companies importing food, dietary supplements, food additives, food and dietary ingredients to the United States must keep pace with changing regulations intended to ensure the safety of U.S. consumer and integrity of the food-based products being imported. FDA has historically seen an import rate increase of 5-10% in the last decade and in 2018, 31% of those were food products requiring prior notice.
Understanding how to navigate the FDA and US Customs import process, including how to utilize their programs to expedite the review of paperwork and shipments at the US ports of entry will be …
Why Safety Data Sheets Are Important for Safe Food Manufacturing Operations
Food Quality and Safety Magazine October 22, 2019 Robert KappEquipment Sanitation May be Co-packer Liability
Gabe Miller, independent consultant and expert in equipment sanitation, published an article in Natural Products Insider on equipment sanitation and the need for co-packers to be aware of how this can impact their business – and liability. “Food processing equipment...Couch Interviewed for Insider Podcast
Senior Director for Dietary Supplement and Tobacco Services, Tara Lin Couch, Ph.D., was interviewed for a Natural Products Insider podcast on how important paper audits are to a contract lab qualification. Couch spoke at the SupplySide West show on this subject in a...FDA Releases Plans for Draft Guidance on the Human Hazard Analysis and Risk-Based Preventive Control (HARPC) Rule – Chapter 14: Recall
FDA announced the release of the next chapter, in draft, of the Guidance for Industry “Hazard Analysis and Risk-Based Preventive Controls for Human Food” on recall plans. This newest chapter will assist the food industry establish and implement a written...FDA Publishes Required Records for FSVP
FDA has published a list of required records for foreign firms exporting food products for both animal and human consumption into the U.S. The Foreign Supplier Verification Program (FSVP), which is part of the Food Safety Modernization Act (FSMA), includes a number of...EAS Discusses Medical Device 510(k) Safety and Performance Measures in ISPE iSpeak Blog
Medical device manufacturers have a new tool available to demonstrate substantial equivalence through FDA’s 510(k) Safety and Performance measures. In a recently released Final Guidance, the Agency, as part of their effort towards stimulating innovation and reducing...Sayler Interviewed for Article on Pathogens and Allergens in Food Safety Strategies
Allen Sayler was interviewed for an article published in Food Safety Strategies on preventing and testing for pathogens and allergens in the food industry including LIMs software and blockchain technologies and internal audits. With an increasing number of food...Safe Foods for Canadians Act (SFCA) Discussed in Food Safety Magazine
EAS Independent Consultant, Ramakrishnan “Rama” Narasimhan published an article in Food Safety Magazine on the Safe Food for Canadians Act, including areas where it is similar to FDA’s FSMA. Did you know EAS offers food safety services for Canadian firms and has a...Dietary Supplements and FSMA Compliance – Fallacy or Fact? – A Complimentary EAS Webinar
Do you know which of the seven major FSMA regulations were designed to support the Food Safety Modernization Act (FSMA)? Join EAS Consulting Group’s FSMA and Dietary Supplement experts Tara Lin Couch, Ph.D., Senior Director for Dietary Supplement and Tobacco Services...FDA’s Approach to Intentional Adulteration
Joe Famiglietti Each month EAS selects one question sent in by readers of EASeNews to answer as part of our Ask the Expert column. This month’s question on how FDA is enforcing Adulterated Foods is answered by Independent Consultant, Joe Famiglietti, who works closely...EAS offers QMS Services for Medical Devices
Quality Management Systems (QMS) for medical devices are cumbersome as layers upon layers of critical checks ensure the safety and effectiveness required for the consumer. However, in an effort to reduce overly burdensome recordkeeping processes FDA has announced a...
Joe Famiglietti
Independent Consultant, Joe Famiglietti, provides guidance to clients regarding FDA compliance matters. He has performed onsite audits at food manufacturing facilities and evaluated production and quality control operations for compliance with FDA regulations. Joe has...Safe Food for Canadians Act Regulations: An Overview
Food Safety Magazine October 15, 2019 https://www.foodsafetymagazine.com/enewsletter/safe-food-for-canadians-act-regulations-an-overview/ Ramakrishnan NarasimhanCo-packer’s equipment may be liability to the brand
Natural Products Insider October 15, 2019 https://www.naturalproductsinsider.com/equipment/co-packers-equipment-may-be-liability-brand Gabe MillerCo-Packer’s Equipment May be Liability to the Brand
Natural Products Insider October 15, 2019 https://www.naturalproductsinsider.com/equipment/co-packers-equipment-may-be-liability-brand Gabe MillerFSVP and Qualified Individual Services
Use of Safety and Performance to Determine Substantial Equivalence – FDA Issues New Guidance for Medical Devices
ISPE iSpeak blog October 7, 2019 https://ispe.org/pharmaceutical-engineering/ispeak/use-safety-and-performance-determine-substantial-equivalence-fda Amy ScanlinPreventing and Testing for Pathogens and Allergens Across the Food Industry
Food Safety Strategies October 4, 2019 https://www.foodsafetystrategies.com/articles/983-preventing-and-testing-for-pathogens-and-allergens-across-the-food-industry Allen SaylerEAS Intern Publishes in FDLI Update Magazine
Neha Mookuparambil, a recent EAS intern focusing on pharmaceutical studies at Georgetown University published an article of FDA’s perspective on continuous manufacturing in FDLI Update, the bi-monthly magazine of the Food Drug Law Institute. EAS has partnered with...
Equipment and Utility Change Control for GMP Production Facilities
By Greg Weilersbacher Despite FDA’s guidance documents on change control, “…managing change to prevent unintended consequences,” many companies limit change control to documentation such as batch records, SOPs, protocols, and specifications and only sporadically...Navigating CBP and Prior Notice of FDA’s Imported Products – October 29, 2019
As the pace of FDA regulated products increases, companies importing food, dietary supplements, food additives, food and dietary ingredients to the United States must keep pace with changing regulations intended to ensure the safety of the U.S. consumer and integrity...Menu Labeling
By Cathryn Sacra Each month EAS selects one question sent in by readers to be answered in EASeNews. This month’s answer is provided by Cathryn Sacra, Director of Labeling and Cosmetic Services. Cathryn oversees EAS’ labeling team, assisting clients with food and...Cirotta Moderates Panel at FDLI Tobacco and Nicotine Product Regulation and Policy Conference
Dean Cirotta, President and COO, is moderating Multi-Stakeholder Reactor Panel at the FDLI 2019 Tobacco and Nicotine Product Regulation and Policy Conference at the National Press Club in DC October 24-25, 2019. The keynote speaker at this event is FDA’s Center for...EAS Offers OTC Labeling Review and Design in Compliance with FDA Monograph Regulations
Did you know that the current regulations for labeling of Over-the-Counter (OTC) drug products were initiated in 1972 as part of the OTC Drug Review? That’s over 50 years ago and the monograph system that arose from that process has not yet come to completion in terms...September 2019 Drug and Device Corner
The FDA has announced the Reorganization of the Office of New Drugs with Corresponding Changes to the Office of Translational Sciences and the Office of Pharmaceutical Quality. This move is one critical component of the agency’s initiative to modernize the New Drugs...Dietary Supplement GMP Enforcement – a Look at Recent FDA Observations and Warning Letters – November 7, 2019
Though FDA’s 21 CFR 111 Good Manufacturing Practices (GMPs) for Dietary Supplements has been in place for over a decade, the industry continues to be plagued by the complexities of compliance. The establishment of specifications for components, in-process materials,...EAS to Exhibit at ISPE Annual Conference Focusing on Pharmaceuticals
Bryan Coleman, Senior Director for Drugs and Medical Devices, and Robert Fish, Independent Advisor for Quality and Compliance will be at the EAS booth, #524, at the International Society of Pharmaceutical Engineers Annual Meeting and Expo October 27-29, 2019 in Las...Tajkarimi Discusses Food Fraud Detection Methods in MeatingPlace
Independent Consultant Mehrdad Tajkarimi, Ph.D. wrote about next-generation sequencing technology as it applies to detect plant and animal species in food adulteration cases for MeatingPlace magazine. NGS produces more variable and more in-depth genome sequencing...EAS Exhibits, Presents Educational Sessions at SupplySide West
If you’ll be in Las Vegas October 15-18, 2019 attending the SupplySide West, stop by EAS booth #5409 and say hello to Tara Lin Couch, Ph.D., Senior Director for Dietary Supplements and Tobacco Products and Independent Consultant Heather Fairman. Both Tara and Heather...
FDA’s Transition from CFR 820 to the ISO 13485:2016 – Instituting a New Quality Management System (QMS)
FDA’s shift to a Quality System Regulation structure of ISO 13485:2016 is an effort to harmonize medical device regulations as well as reduce compliance and record keeping burdens faced by current medical device manufacturers. But will those …
Paper audits important step in contract lab qualification — podcast
Natural Products Insider Podcast September 25, 2019 https://www.naturalproductsinsider.com/labstesting/paper-audits-important-step-contract-lab-qualification-podcast Tara Lin Couch, Ph.D.Food Defense – Untangling the Challenges and Strengthening Opportunities
EAS Independent Advisor for FSMA, Charles Breen and Consultant Kathy Knutson, Ph.D. co-present a webinar on Food Defense. Food Defense is increasingly a worry for firms as our global business climate opens opportunities for intentional harm both domestically and overseas. The ability of food manufacturers …
GRAS Determination: Lessons and Pitfalls
By Tom Jonaitis Over the 14 years of working as a scientific and regulatory consultant in the food industry, I have had the opportunity to work with many companies that were bringing a wide range of exciting and innovative food ingredients to the marketplace in the...What Should We Consider When Exporting Our FDA Regulated Products to the US?
By Victoria Pankovich Each month EAS experts answer one question sent in by readers of EASeNews. This month’s question, on considerations for a reliable US Agent, is answered by Victoria Pankovich, Regulatory Specialist, who assists EAS clients with US Agent...Understanding GRAS Submissions and Avoiding Data Pitfalls- Meet FDA Requirements
Before a substance can be legally added to food in the U.S., with rare exceptions, it must be either an approved food additive or determined to be generally recognized as safe (GRAS) for use in food …
Avoiding Delays of Your Import Cargo Shipments through US Customs and Border Protection (CBP)
By William (Bill) Scopa The US Customs and Border Protection (CBP) has varied enforcement responsibilities, including detecting drug smuggling, weapons of mass destruction, and Immigration. Typically, these concerns are not what will cause the importer delays in their...EAS Assistance Simplifies FDA Registrations, Listings and Renewals
The FDA requires firms that manufacture foods, pharmaceuticals and medical devices to register their facilities on an annual or biennial basis, depending on the product category. Keep in mind, FDA assesses a Medical Device establishment registration user fee annually....EAS to Present Webinar on Prior Notice of Imported Foods for NCBFAA
EAS Independent Consultant Angel Suarez will present a webinar on October 8, 2019 for the National Customs Brokers and Forwarders Association of America (NCBFAA). Discussing the Roles of Import Divisions Review and Compliance, Angel will share insights from his many...Learn More about Infant Formula Considerations in Natural Products Insider
Independent Consultant and infant formula expert, Robbie Burns, Ph.D., published an article in Natural Products Insider on regulatory considerations for the development of infant formula for sale in the U.S. In addition to this article you may wish to review more on...July 2019 Drug and Device Corner
The FDA recently issued a warning letter to a company for inaccurate information in one of their drug listings. Section 510(j) of the Federal Food, Drug, and Cosmetic Act (FD&C Act and 21 CFR Part 207) outlines the requirements for registration and listing of drug...ISPE iSpeak Blog on FDA’s ORA – A Historical Look and Evolving for the Future
Joel Martinez, former FDA ORA BIMO FDA Investigator, and EAS Independent Consultant shared a historical look at this important office and the evolution of training for FDA’s investigators of pharmaceutical products. Learn more here.Determining the Risk-Base of High-Risk Foods
Each month EAS answers one question sent in by readers of EASeNews. This month’s question on the risk-base of high-risk foods is answered by EAS Independent Advisor for FSMA, Charles Breen. Question: How do you determine the Risk-Base of High-Risk Foods? Breen: With...FDA Inspections Webinar with Recent Trends in Observations
EAS has posted our latest webinar to the On-Demand webinars page. Presented by Sophia Lily, Preparing for FDA Inspections, is free to view on-demand – along with numerous other webinars presented by our consultants on a variety of topics. EAS regulatory webinars...Preparing for FDA Inspections, a Look at Recent Observations and Trends
Foreign companies exporting FDA regulated products to the United States can at any time expect an FDA announcement of facility inspection. Looking at the complete safety package from record keeping and retention, to specifications and testing to …
FDA Office of Regulatory Affairs – A Historical Look and Evolving for the Future
ISPE iSpeak blog July 22, 2019 https://ispe.org/pharmaceutical-engineering/ispeak/fda-office-regulatory-affairs-historical-look-and-evolving-future Joel MartinezUS Regulatory Considerations for Infant Formulas
Natural Products Insider July 22, 2019 https://www.naturalproductsinsider.com/regulatory/us-regulatory-considerations-infant-formulas Robert BurnsRisks to OLDs Discussed in Natural Products Insider
Independent Consultant Bruce Elsner discussed considerations of Own Label Distributors for the assurance of GMP compliance and certificates of analysis in Natural Products Insider. Dietary supplement companies that contract out some or all their operations often...Warehousing Compliance and FSMA Reviewed in Food Safety Strategies
The requirement to comply with FSMA is well understood for food manufacturers, and there are no exceptions for warehousing facilities. FDA inspections of facilities that receive, store and distribute human or animal food can occur at any time and firms must be...EAS to Offer Training on Expert Witness Preparations
As highly regulated industries are subject to more claims and litigations, and with growing agency challenges to ingredients, processes and branding, the target company’s regulatory compliance is often a key issue in presenting a successful defense. An expert witness’...How Does USDA Labeling Differ From FDA Food Labeling?
By Susan Glenn Each month EAS experts answer one question sent in by readers. This month’s answer regarding USDA labeling is provided by Susan Glenn. Susan is an expert in matters pertaining to USDA and FDA regulations of the food industry with a particular focus on...Did you know? EAS Consulting Group Offers Preparatory Assessments Against VQIP Requirements for Firms
The Voluntary Qualified Import Program (VQIP) enables qualified importers of food and food products into the U.S. an expedited review and entry. However, meeting the stringent requirements of the VQIP program requires a thorough demonstration of documented safety of...
Rama Narasimhan
Ramakrishnan (aka ‘Rama’) is a versatile, knowledgeable and competent professional with over 35 years of management experience in the manufacturing sectors pertaining to food, pharmaceutical and dietary supplement industries. He has wide ranging experience in...
Robert Kapp, Ph.D.
Robert Kapp, Ph.D. has over 30 years’ experience as a toxicologist involved with the management, development, and safety of new and existing products in a broad spectrum of industries including preclinical program study design, study reports, occupational and...What are SDSs?
By Robert Kapp, Ph.D. Safety Data Sheets (SDSs) (formerly known as Material Data Sheets (MSDSs) contain basic information about a chemical or product needed to insure the safety and health of the user at all stages of its manufacture, storage, use, and disposal....
Angel Suarez
Angel Suarez is a former Supervisor Consumer Safety Officer with the Food & Drug Administration. In this role he had responsibility of import enforcement in areas of seafood and Low-Acid Canned Food (LACF) as well as foreign inspections, warning letters,...June 2019 Drug and Device Corner
As part of the FDA’s ongoing efforts in their goal of more ANDA approvals in order to increase access to high-quality lower cost generic drugs, the agency began on 18 June 2019 to publish additional data in the existing Paragraph IV Patent Certifications list. The FDA...
Challenges with Implementing Cleaning Validation
By Miguel Montalvo Though published well over 25 years ago, FDA’s guidance surrounding cleaning validation continues to cause industry confusion. While everyone agrees that cleaning validation is critical, the application of incorrect or ineffective approaches whether...EAS Offers Pre-Clinical Safety/Tox Study Services for GRAS and NDI Submissions.
EAS offers holistic and cohesive services for clients looking to submit GRAS and NDI submissions to FDA. From the assessments to design of early feasibility studies, ongoing study oversight with the Contract Research Organization, strategy meetings with FDA and the...What are the Steps to Reporting an Adverse Event to FDA?
By Robert Fish Each month EAS independent consultants answer one question sent in by readers of EASeNews. This month’s question on adverse events reporting for both OTCs and dietary supplements is answered by Independent Advisor for Quality and Compliance, Robert...Final Guidance for Food Contact Notifications – Infant Formula and / or Human Milk
FDA’s Final Guidance for preparation of Food Contact Notifications for substances that come into contact with infant formula and human milk is intended to help industry understand FDA’s process for evaluating the safety of food contact substances. It incorporates...Armstrong to Speak at CLE Intro to Food Law
EAS Independent Advisor for Food Law and Regulation, Steve Armstrong, is an invited speaker for the fourth annual CLE Introduction to Food Law conference taking place June 6-7, 2019 at the UCLA Resnick Center for Food Law and Policy. Steve is a third time speaker...EAS Exhibiting at Future Food Tech
EAS Senior Director for Food Consulting Services, Allen Sayler and Independent Consultant, Ron Levine, will represent EAS at Future Food Tech – NYC taking place June 18-19, 2019 in New York City. Stop by the EAS booth to learn more about our services for food...Fairman Authors Supply Chain Transparency and Quality Assurance Articles for Natural Products Insider
EAS Independent Consultant Heather Fairman authored two recent articles for Natural Products Insider. First, Supply Chain Transparency on the demand by consumers and regulators for supply chain transparency from farm to fork. Next, Quality Assurance for...
Miguel Montalvo
EAS Consulting Group independent consultant, Miguel Montalvo, is an expert in GMP and GAP assessments for pharmaceuticals, including injectables, solid dosage, OTC topicals and biologics, as well as medical devices and dietary supplements. He has developed,...
Tom Jonaitis
Tom Jonaitis is a board-certified toxicologist (DABT) that works with clients in the food, dietary supplement, cosmetic, and consumer product industries, providing comprehensive toxicology and regulatory consulting guidance and support. He is an expert in regulatory...EAS Blogs on Proposed Efficiencies in Pharma Manufacturing
EAS regulatory intern Neha Mookuparambil authored a blog for ISPE iSpeak on FDA’s Proposed Approach to Improve Efficiencies for the Advancement of Pharma Manufacturing through Continuous Manufacturing. The FDA has been pushing for advanced manufacturing processes in...FDA Strengthens Process of Initiating Voluntary Recalls
Voluntary recalls are a vital means to protect public health and typically are the quickest way to remove defective or potentially harmful food, medical, and consumer products from the market. FDA’s new Draft Guidance includes steps companies may take to plan, prepare...Final Guidance for Abbreviated Drug Application Submissions
FDA has issued Final Guidance related to drug marketing application submissions for human drugs and recommendations for considerations of whether to submit a 501(b)(2) New Drug Application, commonly referred to as an NDA, or a 505(j) Abbreviated New Drug Application,...Aziz Authors Article on Risk Management in Drug Development and Delivery
EAS independent consultant, Kaiser Aziz discussed FDA’s Quality Risk Management Approach to New Drug Applications in Drug Development and Delivery. Risk management is one of the most important tools in new drug applications to assess the risk level of a drug...EAS to Exhibit at IFT Annual Meeting
Join EAS Chairman and CEO, Ed Steele, President and COO, Dean Cirotta and Director of Labeling and Cosmetics, Cathryn Sacra at the EAS booth #424 on June 3-5, 2019 at the IFT Annual Meeting in New Orleans. Discuss regulatory needs pertaining to foods and dietary...Veronica Ortiz de Montellano
Veronica Ortiz de Montellano Veronica Ortiz de Montellano is based in Mexico and offers assistance in foods, packaging and preservation. She is an expert in food design and process developments including structure, formulation and additives including Thermal Process...Common Pitfalls During Implementation of a Cleaning Validation Program
ISPE iSpeak blog May 30, 2019 https://ispe.org/pharmaceutical-engineering/ispeak/common-pitfalls-during-implementation-cleaning-validation-program Miguel Montalvo10 Key Questions an Own-Label Distributor Should Ask Itself
Natural Products Insider May 28, 2019 https://www.naturalproductsinsider.com/contract-manufacturing/10-key-questions-own-label-distributor-should-ask-itself Bruce ElsnerWarehouse Compliance to the Food Safety Modernization Act
Food Safety Strategies May 23, 2019 https://www.foodsafetystrategies.com/articles/809-warehouse-compliance-to-the-food-safety-modernization-act Jerry HeapsNutrition Bar Manufacturing: A Quality Assurance Perspective
Natural Products Insider May 15, 2019 https://www.naturalproductsinsider.com/regulatory/nutrition-bar-manufacturing-quality-assurance-perspective Heather FairmanFDA and Dietary Supplement Specifications
Tablets and Capsules Solid Dose Digest May 6, 2019 https://www.tabletscapsules.com/enews_tc/2019/issues/tcnews_05_06_19expert.html Tamika CatheySayler Interviewed for Food Processing Magazine Article on Trump’s Effect on Food Industry
EAS Senior Director for Food Consulting Services, Allen Sayler, was interviewed for an article published in Food Processing Magazine on the regulatory state of the food industry under the Trump administration. Published during Scott Gottlieb’s tenure as FDA...
Big Data, Real World Evidence and The Digital Revolution – Hang On!
By: John Brennan Executives from AbbVie, AstraZeneca, Bristol-Myers Squibb, Merck and Co., Johnson and Johnson, Pfizer and Sanofi were grilled on Capital Hill in February on topics ranging from drug pricing, reimbursement, rebates and patent extensions to executive...
John J Brennan Ph.D.
John J. Brennan, Ph.D. is a former Senior Project Leader in Global Pharmaceutical Research and Development at AbbVie in North Chicago, Illinois. At Abbvie he served as the Enterprise Leader for 3 Global Asset Development teams accountable for creating and executing...510(k) Guidance and Substantial Equivalence Discussed in MedTech Intelligence
Independent Consultant, Jay Mansour, discussed the movement away from substantial equivalence in favor of performance testing for the 510(k) application process in a recent MedTech Intelligence. “Expanding on the Abbreviated 510(k) Program for demonstrating...Part-three of Food Safety Training for FSMA Series Published in Food Safety Magazine
Part-three of Dr. Mehrdad Tajkarimi’s series on designing proper employee training programs to ensure FSMA compliance was published in Food Safety Magazine. In this final section, Mehrdad discusses important training parameter matrices as well as development of...Coleman on Opportunities for Pharma Streamlining of Product Innovations in ISPE iSpeak Blog
Senior Director for Pharmaceutical and Medical Device Consulting Services, Bryan Coleman, wrote a blog for the ISPE’s iSpeak on opportunities ahead for the pharma industry as the Agency works to streamline processes for improved innovations.April 2019 Drug and Device Corner
It has come to EAS’s attention that there is significant confusion regarding the exemption of Class 1 Medical Device products to comply with the 21 CFR 801.20 requirement for the label of a medical device to bear a unique device identifier. Per 21 CFR 801.30 A class I...FDA to Begin Inspections for Intentional Adulteration in March 2020
FDA recently announced that verification of compliance with the Intentional Adulteration (IA) rule will begin in March 2020. Addressing hazards that may be intentionally introduced to foods, including by acts of terrorism, with the intent to cause wide-spread harm to...Stewart Discusses Dietary Supplement GMPs in Natural Products Insider
Senior Advisor for Dietary Supplements, Tim Stewart, discussed GMPs in Natural Products Insider. FDA has been asking for additional information beyond GMPs in recent inspections, inquiring on botanical forms and safety. Stewart discusses compliance challenges and best...Insider Podcast on 25 Years of DSHEA Features Senior Director, Tara Lin Couch, Ph.D.
Tara Lin Couch was interviewed for a Natural Products Insider podcast on her reflections of 25 years of DSHEA. Recorded at SupplySide East, she discusses how the dietary supplement industry, pre-DSHEA, was the “wild, wild west” and that 21 CFR 111, Current Good...FDA Proposing to Change the 510(k) Submission Process for Medical Devices
By George Yanulis Each month, EAS selects one question sent in by readers to be answered by one of our experts. This month’s question is answered by George Yanulis D.Eng., an expert in medical device safety and the 510(k) process. Question: Why is FDA proposing to...Final Rule on OTC Hand Sanitizers Issued
FDA issued a Final Rule, effective April 13, 2019, which aims to ensure the safety and effectiveness of OTC hand sanitizers, formally known as topical consumer antiseptic rub products. These products are intended for use without water and marketed under the FDA’s OTC...Did You Know? EAS Offers Verification and Validation Services of Electronic Signatures Under 21 CFR Part 11
Increasing usage of electronic methods to capture and produce critical data, which are subject to regulatory scrutiny led to the effect of Title 21 CFR Part 11. This part of the Code of Federal Regulations establishes the United States Food and Drug Administration...Levine Authors Article on Product Recalls in Natural Products Insider
Independent Consultant Ronald Levine authored an article on the very serious subject of product recalls published in the Natural Products Insider illustrated through a fictitious conversation between a hypothetical company’s CEO and their attorney as they plan for a...FDA Proposed Approach Aims to Improve Efficiencies to Advance Pharma Manufacturing
iSpeak Blog April 30, 2019 https://ispe.org/pharmaceutical-engineering/ispeak/fda-proposed-approach-aims-improve-efficiencies-advance-pharma Neha Mookuparambil
Dennis Gaalswyk
Dennis Gaalswyk assists clients with food safety compliance requirements through development and implementation of FSMA and FSVP protocols. He is an expert in sanitation practices and sanitary equipment, SQF, HACCP and HARPC. Prior to consulting he was a consumer...Supply chain transparency: A practice of trust through legitimacy, from ‘farm to fork’
Natural Products Insider Apr 22, 2019 https://www.naturalproductsinsider.com/supply-chain/supply-chain-transparency-practice-trust-through-legitimacy-farm-fork Heather FairmanSupply Chain Transparency: A Practice of Trust Through Legitimacy, From ‘Farm to Fork’
Natural Products Insider April 22, 2019 https://www.naturalproductsinsider.com/supply-chain/supply-chain-transparency-practice-trust-through-legitimacy-farm-fork Heather Fairman25 Years of DSHEA: A GMP Auditor’s Perspective – Podcast
Natural Products Insider April 18, 2019 https://www.naturalproductsinsider.com/contract-manufacturing/25-years-dshea-gmp-auditor-s-perspective-podcast Tara Lin Couch, Ph.D.Updated Food Safety Training, Solution for Upcoming FSMA Challenges, Part 3
Mehrdad Tajkarimi, D.V.M., M.P.V.M., Ph.D. Food Safety Magazine https://www.foodsafetymagazine.com/enewsletter/updated-food-safety-training-solution-for-upcoming-fsma-challenges-part-3/ April 16, 2019Manufacturing Information Beyond GMPs
Natural Products Insider April 11, 2019 https://www.naturalproductsinsider.com/herbs-botanicals/manufacturing-information-beyond-gmps Timothy Stewart510(k) Guidance Shifts Away Substantial Equivalence in Favor of Performance Testing and Criteria
MedTech Intelligence April 11, 2019 510(k) Guidance Shifts Away Substantial Equivalence in Favor of Performance Testing and Criteria Jay...Product Recall Therapy
Natural Product Insider April 5, 2019 https://www.naturalproductsinsider.com/legal-compliance/product-recall-therapy Ronald J. LevineThe FDA’s Final Link in the Chain of Food Safety and Food Imports
The Food Safety Modernization Act, signed into law in 2011, includes an important provision for food importers under the Foreign Supplier Verification Program (FSVP) Final Rule. Namely, Part 1 Subpart L, Section §1.503 requires that importers of human and animal food enlist a Qualified Individual (QI) who has responsibility for developing a program and …
Regulatory, Technical and Formulations for New Infant Formula Notifications – Challenges and Opportunities
The submission stage of a New Infant Formula Notification may seem like the beginning of the end of a years-long process of research and strategic development. But, without all the right documentation in place, this last step prior to product launch can be unnecessarily delayed for those companies …
Spotlight on Nutraceuticals: Complying with New FDA Requirements for Dietary Supplement Nutrition Labels
Tablets and Capsules April 1, 2019 Spotlight on Nutraceuticals: Complying with new FDA requirements for dietary supplement nutrition labels Gisela...RISK MANAGEMENT – FDA’s Quality Risk Management Approach to New Drug Applications
Drug Development and Delivery April 2019 RISK MANAGEMENT – FDA’s Quality Risk Management Approach to New Drug Applications Kaiser...Preparing For a FDA Preventive Controls Inspection
By Joe Famiglietti The new rule on Preventative Controls for Human Food is mandated by the 2011 FDA Food Safety Modernization Act. Preventive Controls (PC) are steps that a food facility must take to reduce or eliminate food safety hazards. The rule also includes...Armstrong Publishes Article on the Delaney Clause in FDLI Update
Steve Armstrong, EAS Independent Advisor for Food Law and Regulation, is co-author of an article published in the March 2019 Food Drug Law Institute Update Magazine covering FDA’s recent decision to delist six synthetic ingredients as required by the Delaney Clause, a...2019 April Ask the Expert
By Timothy Hansen Each month, EAS answers one question sent in by our readers. This month’s Ask the Expert is answered by Independent Consultant and former head of the NOAA Seafood Inspection Program and Division Director in FDA’s Office of Seafood, Timothy Hansen....
Heidi Stuttz
Heidi Stuttz is an expert in biotech and medical device oversight. She assists clients with a variety of projects including R&D programs, FDA submissions, EMEA dossiers, compliance enhancements and quality improvement initiatives. Assisting with product...A Successful Expert Witness is One Who can Demonstrate Expertise While Connecting with a Jury
An effective Expert Witness is more than one who can write expert opinions, be deposed or provide testimony in court. Experts must represent your company accurately, independently and objectively in matters of legal proceedings and do so in a manner that enables them...RISK MANAGEMENT – FDA’s Quality Risk Management Approach to New Drug Applications
Drug Development & Delivery April 2019 RISK MANAGEMENT – FDA’s Quality Risk Management Approach to New Drug Applications Kaiser J....
Joel Martinez
As a former certified ORA BIMO FDA Investigator, Joel Martinez has completed 20-25 BIMO inspections in all therapeutic areas, interacting with CDER’s Office of Scientific Investigations (OSI) medical reviewers and BIMO Office of Compliance personnel for CBER,...March 2019 Drug and Device Corner
Implementation of reorganization to begin for CDRH In order to create a smart and quick-moving infrastructure that can adapt to the needs of future organizational, regulatory and scientific requirements, the Center for Devices and Radiological Health (CDRH) is...Homeopathy – What Does the Future Hold?
ISPE iSpeak Blog March 4, 2019 https://ispe.org/pharmaceutical-engineering/ispeak/homeopathy-what-does-future-hold Susan CraneFood Additive Reform: Time to Repeal the Delaney Clause?
FDLI Update March 1, 2019 https://www.fdli.org/2019/02/food-additive-reform-time-to-repeal-the-delaney-clause/ Steve ArmstrongHow Do Legal Teams Find and Identify Good Expert Witnesses?
By Ronald J. Levine Each month EAS answers one question sent in by a reader. This month’s question on how to choose an expert witness for FDA legal proceedings is answered by Independent Consultant, Ronald J. Levine. Ron has had a successful career history as a...February 2019 Drug and Device Corner
Sunscreen Innovation Act to Enhance Product Safety Requirements On February 21, 2019 FDA issued a proposed rule that would update regulatory requirements for most sunscreen products in the United States. Aimed at bringing nonprescription, over-the-counter (OTC)...Will FDA Suspend Your Food Facility Registration Based on Environmental Results?
By Kathy Knutson, Ph.D. In October 2018 FDA suspended the registration of Working Cow Homemade, Inc., of Florida. Working Cow is an ice cream manufacturer, ceased operations, and has cooperated with FDA. As part of its decision to suspend their registration FDA...
Janet Collins, Ph.D.
Dr. Janet Collins assists EAS clients with all matters of food regulatory compliance issues. Her expertise includes product development with an eye towards global agricultural advocacy, appreciation for diversity, and strong expertise in human health science,...EAS Webinar: Dietary Supplements and FSMA, Fallacy or Fact?
Since the passage of the Food Safety Modernization Act (FSMA) in 2011, dietary supplements are now subject to many aspects of the seven major regulations that were issued by FDA to support enforcement of FSMA. One of the most important of these regulations being 21...Strategic Product Development a Key Component of Regulatory Compliance
Did You Know? Product development is more than just labeling, it is a holistic approach from ideation through commercialization that strategically looks at the category in which the product is intended to be marketed and those regulatory requirements surrounding it....EAS Webinar – Qualified Individuals – FDA’s Final Link in the Chain of Food Safety and Food Imports
EAS Independent Advisor for Import Operations, Domenic Veneziano and EAS Independent Advisor for FSMA, Charles Breen are collaborating for a complimentary EAS webinar discussing the requirements of a Qualified Individual per FDA requirements. Join us on April 3, 2019,...Sustainable Packaging Discussed in Natural Products Insider
Thomas Dunn has authored an article for Natural Products Insider on sustainable packaging for natural foods. Packaging for natural food products must protect food quality and safety while also connoting clean labels and sustainability, he says.EAS Interview for Article on Food Labeling in Supermarket Perimeter Magazine
EAS Independent Consultant April Kates was interviewed for a recently published article on Food Labeling published in Supermarket Perimeter. EAS is very pleased to have such a strong team of labeling consultants who are often called upon for sharing their...EAS Offers Webinar on Developing New Infant Formula Notification
The submission stage of a New Infant Formula Notification may seem like the beginning of the end of a years-long process of research and strategic development. But, without all the right documentation in place, this last step prior to product launch can be...
Jay Mansour
Jay Mansour is a seasoned Medical Device regulatory consultant with 20+ years of experience. He has successfully filed more than 100 510(k) clearances including De Novo across many technologies and assists clients with QMS turnkey projects, including personnel...
Jeffrey Roberts
Jeffrey Roberts is an expert in software and systems auditing/validation including compliance with 21 CFR Part 11, 21 CFR Part 820 and ISO-13485. He writes Software Development Life Cycle (SDLC) documents including Validation Compliance Plan (VCP), Functional...
George Yanulis, Ph.D.
Dr. George Yanulis has consulted in Medical Device Product Development and Research for 20 years and has a Doctorate and Master’s Degree in Biomedical Engineering. He has conducted cardiovascular device research at the Cleveland Clinic Foundation on cardiac pacing...
Sophia Lily
Sophia Lily has over 25 years of experience in the regulated pharmaceutical, nutraceutical and food industry Quality Control, Quality Assurance. She is based in India and is experienced in handling inspections, validations, vendor audits and training. She routinely...Hemp Products & Confusion Over FDA Remains
Cannabis Industry Journal Hemp Products & Confusion Over FDA Remains February 27, 2019 Charlotte...Food Labeling (Interviewed)
Supermarket Perimeter http://www.nxtbook.com/sosland/sp/2019_02_01/index.php#/70 February 25, 2019 April KatesSustainable Packaging for Natural Foods
Natural Products Insider https://www.naturalproductsinsider.com/co-packing/sustainable-packaging-natural-foods February 19, 2019 Thomas DunnHemp and CBD Regulatory Fact Sheet
EAS Consulting Group Offers Expert Witness Services to Food and Drug Attorneys
In the industries regulated by FDA compliance issues are of great importance. When disputes arise, the regulatory compliance status of a product or facility is often a key issue and can make a significant difference in how the matter will be resolved. In such cases,...Effectively Partnering With a Contract Laboratory (Slide show)
Natural Products Insider https://www.naturalproductsinsider.com/contract-manufacturing/effectively-partnering-contract-laboratory-slide-show February 5, 2019 Tara Lin Couch, Ph.D.EAS Webinar on Regulatory Challenges of Submitting New Infant Formula Notifications
The submission stage of a New Infant Formula Notification may seem like the beginning of the end of a years-long process of research and strategic development. But, without all the right documentation in place, this last step prior to product launch can be...What Does the 2018 Farm Bill Say About Sugar, Honey, and Agave?
By James Hoadley, Ph.D. Each month EAS Independent Consultants answer one question sent in by our readers. This month’s question is answered by James Hoadley, Ph.D., an expert in food and supplement labeling and content claims and long-time instructor for our popular...Natural Products Insider – Unboxing Everything About Packaging
Independent Consultant Thomas Dunn discusses packaging trends in an article for Natural Products Insider. Packaging is an important component of overall product design as it protects, preserves, escorts and describes the products consumed. As consumers demand more...EAS Announces Next GMP Compliance for Laboratories Seminar in Denver
EAS Senior Director for Dietary Supplement Consulting Services, Tara Lin Couch, Ph.D. will instruct the next EAS seminar on Good Manufacturing Practices for Dietary Supplement Laboratories April 23, 2019 in Denver, CO. This one-day intensive program will discuss FDA’s...
Ronald J. Levine
Ron Levine has 40 years of experience advising consumer products companies in complex commercial matters. In addition to providing consulting services for EAS, he serves as the General Counsel of Herrick, Feinstein LLP, a law firm with offices in New York and Newark,...Slack Fill – EAS Experts are Here to Help
There has been an increasingly growing volume of litigation in the slack fill area. This is a daunting issue for food manufacturers and is complicated by a dearth of clear guidance which ultimately leads to a lack of understanding of the many competing requirements...Own Label Distributor Responsibilities Discussed in Natural Products Insider
Senior Director for Dietary Supplement and Tobacco Services, Tara Lin Couch, Ph.D. was interviewed for an article in Natural Products Insider on Own Label Distributors and challenges of industry to establish product specifications. According to FDA data, in fiscal...Food Safety Magazine Part II of Series on FSMA Training
Independent Consultant Mehrdad Tajkarimi has published part two of his three-part series in Food Safety Magazine on designing food safety training programs to meet FSMA compliance expectations. Food safety training is critical, not only in meeting FDA requirements,...January 2019 Drug and Device Corner
With the Federal Register (FR) publication unavailable, the FDA has published Safety and Performance-Based Pathway on the Guidance Document webpage. Full details will be available in the Federal Register once that site is again functioning. The existing Docket Number...Pharmaceutical GMPs for Safer Products and Swifter Approvals
EAS published a blog on the International Society of Pharmaceutical Engineers’ iSpeak blog on how GMPs and data integrity align for safer products and swifter approvals. FDA has noted that in recent years, findings from pharma facility inspections show increasing...Qualified Individual and Preventive Controls Qualified Individual – What’s the Difference?
By Charles Breen The Food Safety Modernization Act (FSMA) Preventive Control for Human Foods (PCHF) regulation (21 CFR 117) signed into law in 2011 offers a wealth of opportunity (and a requirement) for companies to improve their food safety procedures and protocols...How GMPs & Data Integrity Align for Safer Products & Swifter Approvals
ISPE iSpeak blog https://ispe.org/pharmaceutical-engineering/ispeak/how-gmps-data-integrity-align-safer-products-swifter-approvals January 23, 2019 Amy ScanlinOwn-Label Distributors of Supplements Still Failing to Produce Product Specifications
Natural Products Insider https://www.naturalproductsinsider.com/regulatory/own-label-distributors-supplements-still-failing-produce-product-specifications January 22, 2019 Tara Lin Couch, Ph.D.Steps to Develop Compliant SOPs Discussed in AHPA Report
The December American Herbal Products Association (AHPA) Report (subscription required) included an EAS authored article on steps to develop fully compliant Standard Operating Procedures. Though the development and detail of each SOP is at the discretion of individual...FDA Encourages Innovation and Safety as Part of Medical Device Regulatory Overhaul
EAS authored an article in MedTech Intelligence on FDA’s efforts at encouraging innovation while keeping a close eye on safety as part of a medical device regulatory overhaul. FDA is working to retire outdated predicates for 510(k) submissions as well as improve their...Couch Shares Thoughts on Contract Laboratory Best Practices as Part of Insider Q&A
Senior Director for Dietary Supplement and Tobacco Services, Tara Lin Couch, Ph.D. participated in a discussion with other industry leaders on best practices for contract laboratories in a recent Natural Products Insider. Contract labs are often enlisted to certify...2019 Produce Compliance Dates for FSMA
By Charles Breen, EAS Independent Advisor for FSMA Consulting Services January 28, 2019, marks the compliance date for four categories of produce growers: Sprouts from Very Small Farms (with certain exemptions), Sprouts from Very Small Farms eligible for a...Dietary Supplement Specifications Development – Still Challenging the Industry Ten Years Later
By Tamika Cathey Specifications Development, as defined in FDA’s Good Manufacturing Practices for dietary supplements (21 CFR §111) have posed one of the biggest challenges to industry since the inception of the requirements in June 2007. Specifically, 21 CFR §111.70...December 2018 Drug and Device Corner
Guidance Document updates on the FDA website All centers: Developing and Labeling In vitro Companion Diagnostic Devices for a Specific Group or Class of Oncology Therapeutic Products CDER: Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing...FDA De-Listing of Synthetic Flavors
By Steve Armstrong Question: FDA’s recent announcement delisting seven synthetic flavors caused a flurry of conversation and some confusion within the flavor and extract world. Would you clarify? Armstrong: Thank you for the question and the opportunity to clear...
Timothy Morck, Ph.D.
Timothy Morck provides expertise in nutrition-related research, product development, regulatory and public policy and global scientific affairs. Dr. Morck’s career includes clinical nutrition practice, research, and medical school faculty appointments, scientific...Farm Bill Solves “Added Sugar” Problem for Single Ingredient Products
The recently signed Farm Bill answers a number of questions, particularly for those manufacturers of single ingredient foods, jars of honey and maple syrup specifically, who objected to the requirement in FDA’s 2016 Final Rule of adding a declaration of daily value...FDA Technical Amendments for Nutrition and Supplement Facts Panels Released
FDA released a technical amendment correcting or further explaining minor errors and omissions in the May 27, 2016, Final Rules for Food Labeling: Revisions of the Nutrition and Supplement Facts Labels and Food Labeling: Serving Sizes of Foods...Developing Standardized SOPs: The Foundation for Quality Compliance
AHPA Report http://www.ahpa.org/News/AHPAReports/TabId/136/ArtMID/607/ArticleID/1050/Developing-standardized-SOPs-and-updates-on-oils-kratom-and-facilities-registration-in-the-December-2018-AHPA-Report.aspx Amy Scanlin December 18, 2018FDA Encourages Innovation and Safety as Part of Medical Device Regulatory Overhaul
MedTech Intelligence FDA Encourages Innovation and Safety as Part of Medical Device Regulatory Overhaul Amy Scanlin December 7,...FDA’s CDRH Increasing Medical Device Inspections
FDA’s CDRH announced an increasing number of inspections of medical device manufacturers for a targeted risk-based approach for improved compliance in their recent Medical Device Enforcement and Quality Report. Since 2007, the FDA has increased its annual number of...November 2018 Drug and Device Corner
Guidance Document updates on the FDA website: All centers: Post-Complete Response Letter Meetings Between FDA and ANDA Applicants Under GDUFA Guidance for IndustryOn December 3, 2018, the U.S. Food and Drug Administration (FDA) published the guidance for industry...Overcoming Seafood Fraud – a Free EAS Webinar Presented by Tim Hansen
Seafood is largely accepted based on how it is labeled or listed on a menu. This is true for seafood wholesalers, distributors as well as retailers. The financial incentive for unethical practices is huge but measures can be adopted to protect product identity and...Essential Quality Systems for Own Label Distributors Discussed in Natural Products Insider
Senior Director for Dietary Supplement and Tobacco Services, Tara Lin Couch, Ph.D., has written an article for Natural Products Insider on the intersection of OLD and Quality Systems. “The development and implementation of essential own label distributor quality...Appendix T Storm is Hitting the Grade A Dairy Industry
Senior Director for Food Consulting Services, Allen Sayler, published an article in Dairy Foods Magazine on FDA’s PMO (Produce Milk Ordinance) new Appendix T, which mirrors FSMA in many ways in that it requires preventive controls for those hazards not already covered...New Report on the Sources of Foodborne Illnesses Highlights Urgency of FSMA Compliance
By Charles Breen, Independent Advisor for FSMA The Interagency Food Safety Analytics Collaboration (IFSAC) report, “Foodborne illness source attribution estimates for 2016 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter using...Laser Products and 510(k) Requirements
By Jerry Dennis Each month EAS’ Ask the Expert answers questions sent in by readers on a variety of FDA regulatory topics. This month’s question on FDA’s regulation of lasers and 510(k) applications is answered by Jerry Dennis. Jerry is a former member of CDRH where...FDLI Cannabis Conference Covers Possible Pathways to Regulation
The first annual FLDI Cannabis Conference was held in Washington D.C. on November 2nd. What made this cannabis conference unique for me was not the content or the speakers but the audience attending. I have been part of the cannabis industry for the past four years,...Contract Labs Best Practices
Natural Products Insider https://www.naturalproductsinsider.com/labstesting/contract-labs-best-practices Tara Lin Couch, Ph.D. November 27, 2018The Appendix ‘T’ Storm is Hitting the U.S. Grade ‘A’ Dairy Industry
Dairy Foods Magazine https://www.dairyfoods.com/blogs/14-dairy-foods-blog/post/93262-the-appendix-t-storm-is-hitting-the-us-grade-a-dairy-industry Allen Sayler November 27, 2018Essential Quality Systems for an Own Label Distributor
Natural Products Insider https://www.naturalproductsinsider.com/contract-manufacturing/essential-quality-systems-own-label-distributor Tara Lin Couch, Ph.D. November 2, 2018Mutual Recognition Agreements for Monitoring US Drug Supply Written for Tablets & Capsules
James Evans expanded on his September 2018 issue of the month article on MRAs in an article published in Tablets and Capsules magazine. He discusses MRAs in-depth, as well as Field Action Reports, Med-Watch Reports, and Drug Quality Sampling and Testing as methods of...EAS to Present Two Sessions at Food Safety Consortium
EAS will be presenting two sessions at the upcoming Food Safety Tech’s Food Safety Consortium taking place November 13-15, 2018 in Schaumburg, IL. Independent Consultant Andrea Yablunoksy will present on Recall Readiness: Understanding Requirements, Similarities &...How to Prioritize Planning for Food Safety Emergencies
By Charles Breen This month’s Ask the Expert is on how to prioritize planning for food safety emergencies, particularly in light of the challenges of not only the emergency itself but recovering from it with your company’s reputation intact. It is answered by Charles...Overcoming Seafood Fraud – a Free EAS Webinar Presented by Tim Hansen
Seafood is largely accepted based on how it is labeled or listed on a menu. This is true for seafood wholesalers, distributors as well as retailers. The financial incentive for unethical practices is huge but measures can be adopted by processors, wholesalers, and...FDA Proposes Releasing Retailer Names in Most Serious Recalls
By Charles Breen, Independent Advisor for FSMA In an effort at greater transparency as well as consumer safety, FDA issued Draft Guidance this month proposing how and when FDA would publicize the identities of retail consignees that may have received recalled human or...EAS Consultants Present FSVP Webinar for NCBFAA
EAS Independent Advisor for FSMA, Charles Breen and EAS Independent Consultant, Bryan Armentrout are presenting a webinar focusing on FSVP requirements for the National Customs Brokers and Forwarders Association, NCBFAA. Titled “Foreign Supplier Verification and How...Four Common Mistakes When Selecting a Rapid Method for Environmental Testing
By Bryan Armentrout We are now firmly in the era of the Food Safety Modernization Act. It’s no longer theoretical, it’s real world, and FDA is auditing and asking questions about what they are seeing. I think we can all agree on one thing. The way we looked at...Crozier-Dodson Article on Pathogen Control in Poultry Processing Published in Meating Place
Beth Crozier-Dodson published an article in Meating Place discussing top tips for proactive pathogen control in poultry processing. “One of the most significant aspects of any poultry food safety plan is the implementation of pathogen control measures at each critical...Own Label Distributor Responsibilities Discussed in Natural Products Insider
Senior Director for Dietary Supplement and Tobacco Services, Tara Lin Couch, Ph.D. discussed challenges facing Own Label Distributors in the Natural Products Insider. Although a firm may contract certain dietary supplement manufacturing operations, it cannot contract...Good Food Movement Discussed in Natural Product Insider
Independent Advisor for Food Law and Regulation, Steve Armstrong, JD, has written an article on the Good Food Movement for Natural Products Insider. “Consumers are increasingly looking for foods with clean labels,” he says. Unfortunately, there is no regulatory...Photos of Old Town Alexandria Put Final Touch on EAS Office Expansion
EAS Headquarters has just undergone a renovation to add additional space to its headquarter offices in the heart of Old Town Alexandria. Included in the buildout is more office space to accommodate expanding staff and the inclusions of a larger conference and training...Being a Third-Eye in a Plant with a Problem – Discussions on Listeria and Salmonella Protection in Food Safety Magazine
Carl Custer wrote about the challenges of Listeria, Salmonella, extraneous materials and worse in an article published in Food Safety Magazine. Carl also shared some of his experiences at problem-solving both while at FSIS and working with clients as a consultant,...Medical Device Regulatory Landscape Discussed in Post AdvaMed MedTech Wrap Up
Senior Director for Pharmaceuticals and Medical Devices, Bryan J. Coleman discussed innovations and the regulatory landscape in an AdvaMed MedTech blog. Bryan shared his thoughts as FDA continues to harmonize and leverage existing regulatory approaches in increasingly...Pharma’s Problems with Data Integrity
FDA takes data Integrity very seriously and their many Warning Letters and Import Alerts to dosage form and Active Pharmaceutical Ingredient (API) manufacturers in the past several years, indicate that validation is a consistent problem for the pharmaceutical industry. Data integrity, or lack thereof …
Being a Third Eye in a Plant with a Problem
Food Safety Magazine https://www.foodsafetymagazine.com/enewsletter/being-the-third-eye-in-a-plant-with-a-problem-listeria-salmonella-extraneous-materials-or-worse/ Carl Custer October 16, 2018EAS Presents at United Nations to SIDS DOCK Assembly
For Immediate Release EAS Consulting Group, LLC Alexandria, VA 571-447-5500 easconsultinggroup.com October 5, 2018: EAS Consulting Group, LLC Announces Collaboration with Small Islands and Presents on FDA Regulatory Services to Fourth Session of the SIDS DOCK Assembly...FDA Announces Export Certifications and Fees for Certain Food Products
By Charles Breen, Independent Advisor for FSMA On August 31, 2018, FDA announced a new voluntary export certification option for certain foods as authorized under FSMA, similar to that available for qualified pharmaceuticals and medical devices. This new export...Fairman Discusses Tips to Ensure Compliance with Audits in Natural Products Insider
EAS Independent Consultant Heather Fairman discussed Own Label Distributor audits of contract manufacturers as well as steps for ensuring their compliance in the Natural Products Insider. “Own label distributors performing contract supplier and/or contract...Couch Shares Dietary Supplement OLD Responsibilities in AgroFOOD Industry HI Tech
Senior Director Tara Lin Couch, Ph.D. shared Own Label Distributor (OLD) responsibilities in a recent article published in Technoscienze – AgroFOOD Industry HI Tech. Regardless of whether an OLD is domestic or international, the FDA is clear that the OLD is...Skolnik Discusses AERs in Natural Products Insider
Norma Skolnik discussed Adverse Events Reporting (AER) requirements for the dietary supplement industry in Natural Products Insider. The requirements as well as the Guidance for Industry: Questions & Answers Regarding Adverse Event Reporting and Recordkeeping for...Cirotta to Moderate Panel at FDLI Conference Focusing on Tobacco and Nicotine Products
Dean Cirotta will moderate a panel at the upcoming FDLI Tobacco and Nicotine Products Regulation and Policy Conference concerning “The Latest on Product Standards and Other Potential Regulatory Action”. The conference, which takes place October 25-26, 2018 in...Breen Co-Authors Article in Food Quality and Safety Magazine on Responding to Food Safety Emergencies
Independent Advisor for FSMA, Charles Breen has co-authored an article with Stacey Stevens, Senior Vice President of FoodMinds, a food PR firm with which EAS has a collaborative partnership on responding to a food safety emergency. The article, published in Food...Legislation to Reform the OTC Drug Monograph System: The OTC Drug Safety, Innovation, and Reform Act
By Norma Skolnik, Independent Consultant With the aim of overhauling the regulation of over-the-counter (OTC) monograph drugs, U.S. Senators Johnny Isakson and Bob Casey recently sponsored bipartisan legislation, the Over-the-Counter Drug Safety, Innovation, and...Top Tips for Pathogen Control in Poultry Processing
MeatingPlace http://www.meatingplace.com/Industry/TechnicalArticles/Details/80524 Beth Crozier-Dodson October 1, 2018The Good Food Movement Transforms Food Labeling
Natural Products Insider https://www.naturalproductsinsider.com/foods/good-food-movement-transforms-food-labeling Steve Armstrong, JD October 1, 2018Mututal Recognition Agreements and Other FDA Activities for Monitoring the US Drug Product Supply Chain
Tablets and Capsules https://www.e-digitaleditions.com/i/1035980-tc1018/13? James Evans October 2018September 2018 Drug and Device Corner
EAS would like to remind clients, the FDA FY 2019 establishment registration renewal period begins this month. With reference to Drug Establishments, please make sure you are aware of your obligations under the Drug Supply Chain Security Act (DSCSA) during this drug...Ensuring a Contract Laboratory is in Compliance
Natural Products Insider https://www.naturalproductsinsider.com/labstesting/ensuring-contract-laboratory-compliance Tara Lin Couch, Ph.D. September 26, 2018Are Industry Initiated Environmental Swab-a-Thons a Benefit?
The emphasis on proactive management of issues that could cause a food safety hazard, per FDA’s Food Safety Modernization Act, requires firms to improve controls for a variety of issues at all levels. One hot-button concern that continues to wreak havoc in …
Dietary Supplements Own Label Distributor (OLD) Regulatory Responsibilities: Six Critical Items and Processes
TechnoScienze Dietary supplements Own Label Distributor (OLD) regulatory responsibilities: six critical items and processes Tara Lin Couch, Ph.D. September 4,...What Exactly is the Foreign Supplier Verification Program (FSVP)?
By Allen Sayler Ask the Expert offers a chance for our readers to submit questions to EAS regarding areas of regulatory confusion. This month’s question is answered by Allen Sayler, Senior Director of Food Consulting Services. If you’d like to submit a question,...Couch Discusses Contract Manufacturing Partnerships in Natural Products Insider Podcast
Senior Director for Dietary Supplements, Dr. Tara Lin Couch was interviewed for a Natural Products Insider podcast on contract manufacturing partnerships. Dr. Couch is one of two EAS presenters for the upcoming SupplySide West trade show taking place in Las...Tajkarimi and Sayler Discuss RTEs in Natural Products Insider
Independent Consultant Mehrdad Tajkarimi and Senior Director Allen Sayler co-wrote an article for Natural Products Insider discussing Cause, Effect and Impact of RTE Operations on complying with FDA’s draft Listeria guidance. Food manufacturers are expected to operate...Preventive Controls – A New Ballgame for Food Safety Compliance
By Charles Breen, Independent Advisor for FSMA With the recent issuance of a Warning Letter citing violations of Part 117 subpart C (preventive controls), implementation of FDA’s Preventive Controls rule takes its next step – FDA will cite food facilities for...
James Evans
James Evans has more than 30 years of experience as an FDA auditor and specializes in pharmaceuticals, medical devices, biologics, and biotechnology. His expertise includes pharmaceutical inspections, antibiotics, radiopharmaceuticals, parenteral, sterilization,...
FDA Mutual Recognition Agreements
By James Evans, EAS Independent Consultant FDA has been implementing the Safety and Innovation Act since it was passed by Congress in 2012. The Act requires FDA to establish Mutual Recognition Agreements (MRAs) which are agreements between two or more countries to...Sayler Discusses Fast Tracking FDA FURLS in Food Safety Tech
Senior Director for Food Consulting Services, Allen Sayler, has written an article for Food Safety Tech on FDA Fast-Track Unified and Listing Systems (FURLS) program and whether it is expediting or impeding access to overseas markets. “The FDA recently released a...Tajkarimi Writes of Food Safety Training as a Solution for FSMA Challenges
Mehrdad Tajkarimi discusses the importance of food safety training as a solution to FSMA challenges in Part One of a three-part series for Food Safety Magazine. “There are several challenges ahead for proper food safety training at all levels,” he says. “These...Lavieri Highlights the Impact of the Human Element in Food Safety Programs in Food Safety News
Robert Lavieri authored a guest column in Food Safety News on the important and often overlooked “human element” to food safety planning and programs. Companies spend much time and effort designing and cross-checking procedures to protect against food safety issues,...Adverse Events Reporting for Dietary Supplements
Natural Products Insider https://www.naturalproductsinsider.com/regulatory/adverse-event-reporting-requirements-dietary-supplements Norma Skolnik August 29, 2018How to Survive your Worst Food Safety Nightmare
Food Quality and Safety How to Survive Your Worst Food-Safety Nightmare Charles Breen August 29,...Temporary Marketing Permits – Opportunity and Pitfalls of the Specialized FDA Application
Food Standards are an important component of FDA’s oversight, ensuring honesty and fairness to the consumer through requirements that provide for the basic nature of a standardized food to be uniform in terms of its characteristics as well as the ingredients that it must or may contain, (i.e., mandatory and …
Fast Track to FDA FURLS—Expediting or Impeding Access to Overseas Markets
Food Safety Tech https://www.naturalproductsinsider.com/contract-manufacturing/supplyside-west-podcast-quality-systems-contract-manufacturing-partnership Allen Sayler August 14, 2018Podcast Interview: Quality Systems in a Contract Manufacturing Partnership
Natural Products Insider https://www.naturalproductsinsider.com/contract-manufacturing/supplyside-west-podcast-quality-systems-contract-manufacturing-partnership Tara Lin Couch, Ph.D. August 8, 2018Chilean Salmon
Science Direct – Food Regulations & Enforcement
European Contract Manufacturers: Preparing for US client compliance expectations
Ask An Expert: Managing the quality of your contract manufacturer
Have a Plan Before FDA Arrives
Auditing a Contract Laboratory
Infographic: Validating a Contract Laboratory
Evolution of FDA Regulatory of Dietary Fiber Substantiation
Dietary Fiber Definition Challenges
Color Coding: Adding Discipline to a Food Processor’s Preventive Control Program
Food Defense Training and the New “Focused Mitigation Strategies to Protect Food against Intentional Adulteration” Rule
Spotlight on Nutraceuticals: FDA draft Guidance on new dietary ingredients: Do you need to file?
Audits Help Build Successful Contract Manufacturing Partnerships
Video Interview: The Potential Delay to Nutrition Facts labels should not slow compliance efforts
The Impact Workplace Safety Programs Have on Company Culture
Keeping ‘Clean Label’ Claims Legal
Updated Food Safety Training, Solution for Upcoming FSMA Challenges
Food Safety Magazine https://www.foodsafetymagazine.com/enewsletter/updated-food-safety-training-solution-for-upcoming-fsma-challenges/ Mehrdad Tajkarimi August 7, 2018Botanical Submissions: NDI Versus GRAS in a Post-DSHEA World
AHPA Report http://ahpa.org/News/AHPAReports/TabId/136/ArtMID/607/ArticleID/1013/In-Florida-the-berries-have-hit-the-fan-in-the-August-2018-AHPA-Report.aspx Amy Scanlin August 7, 2018Quality Systems for the Cannabis Industry – Preparing for State GMP Regulations
As states begin to regulate legalized cannabis, the concern of how Good Manufacturing Practices (GMPs) apply to this unique industry cannot be understated. While regulations vary from state to state the quality systems under which cannabis products are grown and manufactured have similarities that can begin to pave the way for …
FDA 483 Responses – Missing the Mark?
By Cindy Beehner, Independent Consultant In recent months, it appears more companies are having difficulties meeting the requirements of FDA Form 483, List of Observations, response. The inspection situation is very stressful and if it ends with the dreaded “483”, it...Are Plant-Based Proteins Milking Dairy?
By Allen Sayler, Senior Director for Food Consulting Services When FDA announced recently that the agency is seeking public comment for standards of identity overhaul that will focus in part on plant-based products that are marketed as “milk” substitutes,...EAS Co-Authors Article for FDLI Update on the Benefits of Consultants as Part of a Legal Team
EAS joined forces with Ronald J. Levine, co-chair on Herrick, Feinstein LLP’s Litigation Department to author an article published in the Food Drug Law Institute Update Magazine on how the use of consultants can benefit a legal team. Consultants provide unique...EAS Consultants Invited Speakers at Two FDLI Conferences
EAS is pleased to be invited speakers at two upcoming Food Drug Law Institute (FDLI) Events. Andrea Yablunosky will speak at an in-house event held at FDA CFSAN on Food Labeling: Nutrient Content, Health, and Other Claims on August 7th. Bruce Silverglade will speak on...EAS Offers Complimentary Webinars on Cannabis, Foreign FDA Inspections, Temporary Marketing Permits and More
Quality Systems for the Cannabis Industry – Preparing for State GMPs August 6, 2018, 1:00pm Eastern Tara Lin Couch Ph.D., Senior Director for Dietary Supplements and Tobacco Services, will help cannabis firms prepare for cannabis Good Manufacturing Practices (GMPs)....New Webinar on Environmental Monitoring Added to EAS’ Summer Compliance Line-Up
Are Industry-Initiated Environmental Swab-a-Thons a Benefit? September 17, 2018, 1:00pm Eastern The emphasis on proactive management of issues that could cause a food safety hazard, per FDA’s Food Safety Modernization Act, requires firms to improve controls for a...Cause, Effect and Impact of RTE Operations on Complying With FDA’s Draft Listeria Guidance
Natural Products Insider https://www.naturalproductsinsider.com/supply-chain/cause-effect-and-impact-rte-operations-complying-fdas-draft-listeria-guidance Mehrdad Tajkarimi July 31, 2018Factoring in the Human Element in Food Safety Programs
Food Safety News Factoring in the human element in food safety programs Robert Lavieri July 28,...Produce Safety Rule’s July Compliance Dates
By Charles Breen, Independent Advisor for FSMA Those closely watching FSMA compliance dates will note that July 26, 2018 marks a significant date for FSVP and the Produce Safety Rule. Specifically, importers with Small Business Foreign Suppliers will now be required...EAS Consultants to Present in Two Technical Sessions at the IFT 2018 Annual Meeting
EAS Independent Advisor Charles Breen is the panel moderator for two technical sessions at IFT – one on the Preventive Controls for Human Foods and another on the Foreign Supplier Verification Program. Esteemed panelists include for PCHF, Joann Givens, Director of the...EAS Short Videos Describe Services to the Food Industry
EAS offers a wealth of regulatory consulting capabilities in all FDA commodity areas, as well as USDA and some state regulatory services as they pertain to food and dietary supplement products. EAS is creating short clips that discuss our capabilities and is posting...Probiotics Here to Stay Says DeMuri in Natural Products Insider
EAS Independent Consultant Steve DeMuri authored an article in Natural Products Insider on Probiotic health benefits of natural foods and supplements. With many reported health benefits currently being studied, “Probiotics are here to stay and now is the time for...Elizabeth Campbell and April Kates Discuss Clean Labels in Natural Products Insider
EAS Independent Advisor Elizabeth “Betty” Campbell and Independent Consultant, April Kates have co-written an article on how to keep “clean label” claims legal for Natural Products Insider. “Marketing a clean label can be a great way to for brands to connect to...Kristen Steel
Kristen Steel is a regulatory professional with a strong background in healthcare marketing, compliance and strategic partnerships within healthcare, CPG, and foodservice environments. She works with project and database management, organizational management,...
Philip Scharago
Philip Scharago is a pharmaceutical consultant with extensive knowledge of ISO 13485, 14971, QSR GMP 820, ICH, MDD/EU, ICH, CGMP, Quality Systems, Risk-Based Auditing, process validation, and equipment qualification for pharmaceutical manufacturing and testing. He has...Food Safety Plan Builder Updates Released
FDA has released an updated version of the downloadable Food Safety Plan Builder tool designed to help owners and operators of food establishments with the development of a food safety plan that identified hazards requiring preventive controls to prevent foodborne...FDA Food Code 12th Edition – What’s New?
By Charles S. Otto, III, Independent Consultant The FDA Food Code is used as the basis for food safety regulation of more than a million restaurants, retail food stores, institutional and other food operations in the US and around the world. It is updated every two...OTC Monograph System Gets an Update
By Susan Crane This month’s Ask the Expert is answered by EAS Independent Advisor for OTC Drugs and Labeling, Susan Crane. Susan specializes in quality and regulatory compliance for over-the-counter (OTC) and dietary supplement products. She has a thorough knowledge...Schebella Offers Tips for Designing Cannabis Food Edibles in the State of California in Cannabis Industry Journal
Independent Consultant Celia Schebella discusses tips for designing a cannabis edible in the state of California that meets customer expectations and regulatory requirements in Cannabis Industry Journal. Designing a cannabis food product with GMPs, local regulations,...Updated Guidance for Declaration of Dietary Fiber in Nutrition Labels
Before 2016, FDA regulations for nutrition labeling did not define the term “dietary fiber” but in 2016, FDA issued regulation defining dietary fiber as two types of fiber: [1] non-digestible soluble and insoluble carbohydrates and lignin that are intrinsic and intact...June 2018 Drug and Device Corner
Federal Register Notice Vol 83, No. 108 FDA is publishing an order to exempt a list of class II devices from premarket notification (510(k)) requirements, subject to certain limitations. This exemption from 510(k), subject to certain limitations, is immediately in...Designing the Perfect Cannabis Edible in California
Cannabis Industry Journal Designing the Perfect Cannabis Edible in California Celia Schebella June 28,...Conferencia web de EAS: Programa de verificación de proveedores extranjeros – ¿qué significa para usted?
Si usted exporta alimentos, ingredientes, empaques, suplementos o aditivos alimenticios hacia los Estados Unidos, su agente aduanal en los Estados Unidos, importador y clientes le pedirán proporcionar documentación para verificar que su empresa cumple con la Ley de Modernización de la …
Being Prepared – Critical Points to an FDA Inspection
Tobacco Reporter https://www.tobaccoreporter.com/2018/06/being-prepared/ Karen Dixon June 1, 2018The Supply Chain and Food Safety Culture: Distribution
Food Safety Magazine https://www.foodsafetymagazine.com/magazine-archive1/junejuly-2017/the-supply-chain-and-food-safety-culture-distribution/ Veny Gapud June/July 2017Blomquist to Present on Rapid Testing Methods at the IAFP
Independent Consultant, David Blomquist, will speak on “Rapid Testing Methods for Safety and Spoilage in the Dairy Industry – What Is Needed, What Works and What Does Not” on July 9, 2018, at the International Association of Food Protection Conference. Says...Armstrong Authors Article on Mergers and Acquisitions for Natural Products Insider
Independent Advisor for Food Law and Regulation, Steve Armstrong, has authored an article for Natural Products Insider on buying a 21st-century food company and mergers and acquisitions. “A health and nutrition startup should resolve any potential issues with its...Dietary Supplement GMP Online Short Course Available for Purchase
EAS Senior Director for Dietary Supplement and Tobacco Services, Tara Lin Couch, Ph.D., along with attorney Marc Ullman from Rivkin Radler, and EAS Independent Advisor for Quality and Compliance, Robert Fish, held a Dietary Supplement Short Course, consisting of four...May 2018 Drug and Device Corner
In the ongoing effort to make more affordable drugs available to the public, FDA Commissioner Scott Gottlieb, MD issued a statement on the FDA’s efforts to assist in this process. Of particular note is the FDA’s commitment to ensuring generic drug developers have...New Brewers Need to Know GMPs
By Charles Breen, Independent Advisor for FSMA The explosion in the numbers of new breweries is a blessing for beer drinkers and their communities. In FDA’s eye, beer is food, and while exempt from preventive control requirements, brewers must comply with good...EAS to Moderate Two Panels at IFT Annual Meeting, Exhibits at booth #S322
EAS will be well represented at the upcoming IFT Annual Meeting taking place in Chicago, July 15-18, 2018. EAS Chairman and CEO, Ed Steele, President and COO, Dean Cirotta and Senior Director for Food Consulting Services, Allen Sayler, will be manning the EAS booth...EAS Authors Article in MedTech Intelligence on the New De Novo Pathway
EAS authored an article for MedTech Intelligence on FDA’s new approach to the regulatory pathway for De Novo medical device classification which simplifies the approval process for the class I or class II devices for which there is no one-to-one precedent or...Why is Codex Alimentarius Important to Me?
By Allen Sayler This month’s Ask the Expert is answered by Senior Director for Food Consulting Services, Allen Sayler, who recently returned from the 50th session of the Codex Committee on Food Additives held in Xiamen, China where 53 countries and 32 food industry...USDA Announces Members of NACMCF – Two EAS Independent Consultants are Included
The names of new and returning members of the National Advisory Committee on Microbiological Criteria for Foods (NACMCF) have been announced and the committee now includes two EAS Independent Consultants. Returning member, Dr. Omar Oyarzabal who is a professor at the...FDA Expands Data Dashboard to Include FSMA
A new section of the FDA Data Dashboard has been launched to help importers and manufacturers/processors meet supply-chain requirements under the FDA Food Safety Modernization Act (FSMA) by helping them more easily find compliance and enforcement information related...Medical Device Innovations and the Regulatory Landscape
By Dawn Wydner, Independent Consultant I’m amazed as I watch a Health Care System and then a Spectrum commercial to what our future holds with the sophisticated, interactive medical device and technology innovations and how they will enhance the capabilities of...EAS to Exhibit at Food Defense Conference
EAS will be exhibiting at the upcoming fourth annual Food Defense Conference to be held May 22-24, 2018 in Minneapolis. Senior Director for Food Consulting Services Allen Sayler will be in attendance along with experts from around the world to discuss and learn from...
Richard White
Richard White is a former Director of Codex and International Standards Policy at the Grocery Manufacturers Association. Prior to GMA, he had a career serving US public interests as the Senior Director, Sanitary and Phytosanitary Affairs at the Office of the US Trade...CTP Updates Provisional Substantial Equivalence Review Process
EAS Consulting Group would like to bring to your attention an FDA Center for Tobacco Products announcement regarding updates to the Provisional Substantial Equivalence review process. Substantial equivalence (SE) is the most commonly used pathway by which tobacco...Tara Lin Couch, Ph.D. to Discuss Preparing for Cannabis GMPs
Tara Lin Couch Ph.D., Senior Director for Dietary Supplements and Tobacco Services, will present a webinar on August 6, 2018, at 1:00 pm Eastern to help cannabis firms prepare for cannabis Good Manufacturing Practices (GMPs). As states begin to regulate legalized...Gabe Miller Discusses Necessity of Food Safety Programs for the Cannabis industry
Gabe Miller, an expert in Food Safety Programs has written an article for the Cannabis Industry Journal on how solid food safety programs can help make cannabis products safer and save businesses a lot of time and money. Gabe spoke at the upcoming University of...Fairman Authors Articles on Workplace Safety and Golden Rules for Co-Packers
Independent Consultant, Heather Fairman has published two articles recently. One in Food Processing Magazine focuses on the positive impact of workplace safety programs on corporate culture. Next, she wrote about five “golden rules” for co-packers to stay competitive...Steve Murphy to Present on Raw Materials at ADSA in Knoxville
Steve Murphy will present a session called “Time for Change; Indicators of Public Health Concern for Raw Milk and Processed Dairy Products” at the upcoming American Dairy Science Association Annual Meeting taking place June 24-27, 2018 in Knoxville. Says Murphy,...EAS Independent Experts Offer Summer Webinar Compliance Series
EAS Consulting Group is offering a full line-up of educational opportunities through our informative webinar series. These 45-minute webinars are an opportunity to get up to the minute regulatory information on the latest topics pertaining to FDA regulated industries,...FDA Confirms to Congressional Representatives that Safety is the Number One Priority for Dietary Supplements
FDA recently responded to a letter from congressional representatives regarding product safety in the dietary supplement industry. In the letter, dated April 12, 2018, the agency states that safety, product integrity, and informed decision-making are the...April 2018 Drug and Device Corner
Reminder! 5 May 2018 is the date for DMF (Drug Master File) and IND (Investigational New Drug) applications to comply with eCTD format submission. There has been a delay for Type III DMF files to comply. The FDA announced in April via their Guidance Document Providing...Clean Label, the Challenges
By Gustavo M. Gonzalez, Ph.D. So, you decided to have clean labels for your products, now what do you do? You are not alone in your pursuits of this endeavor. A couple of years ago, I was approached by a marketing department when they wanted to make certain claims in...Guidance for Industry on Dietary Supplement Products Containing Highly Concentrated Caffeine
FDA released Guidance for Industry on highly concentrated caffeine in dietary supplements. FDA considers products which only or primarily consist of pure or highly concentrated caffeine and are sold as dietary supplements to be adulterated under section 402(f)(1)(A)...
Dawn Wydner, Ph.D.
Dawn Wydner, Ph.D., consults in pharma and medical devices, providing proactive compliance and application of quality oversight in all aspects of operational strategy, coordination and conduct. Prior to consulting she was the Senior Director of BioResearch, Quality,...How can implementing a GMP system for regulatory compliance also streamline business at a cannabis facility?
By Kathy Knutson This month’s Ask the Expert is answered by Independent Consultant, Kathy Knutson, Ph.D. Kathy is a lead instructor for Preventive Controls for Human Food (PCHF); Preventive Controls Qualified Individual (PCQI) and trained in the prevention of...Nutrition Facts and Supplement Facts Labels Compliance Dates Extended
The U.S. Food and Drug Administration has issued a final rule to extend the compliance dates for updating Nutrition Facts and Supplement Facts labels, from July 26, 2018, to January 1, 2020, for manufacturers with $10 million or more in annual food sales....Inflation Adjusted Values for Six FSMA Regulations
The FDA released inflation-adjusted values for six FSMA regulations covering 2016-2017. These values are particularly noteworthy for smaller businesses that may not be covered, may receive an exemption, or have later compliance dates based on their sales being less...CTP Announces Actively Working Tobacco Product Manufacturing Practices
At TMAs 103rd Annual Meeting held on Tuesday, April 10, 2018, Dr. Zeller confirmed that FDA is actively working on a number of priorities that he called “Foundational Rules and Guidances”. This includes the issuing of the Tobacco Product Manufacturing Practice (TPMP)...Independent Advisor for Food Law and Regulation, Steve Armstrong, Wins Service to FDLI Award
Steve Armstrong is the winner of the 2018 Service to FDLI award, presented at this year’s FDLI Annual Conference held in Washington, D.C. Steve is the former chief law counsel at Campbell Soup Company and has been consulting with EAS, providing expert opinion and...Sayler Presents on 21 CFR 117 at the AHPA Dietary Supplement Regulatory Summit
Senior Director for Food Consulting Services, Allen Sayler, will speak on how FSMA impacts the dietary supplement industry during the Dietary Supplement Regulatory Summit, taking place May 16, 2018, in Washington, DC. The Dietary Supplements Regulatory Summit is the...
Andrea Yablunosky
Andrea Yablunosky’s focus is product development, labeling compliance, and risk mitigation for issues pertaining to USDA/FSIS. With a background in food science and nutrition she is well-versed in policy development, product reformulations and promotions, recall...Probiotic Health Benefits in Natural Foods and Supplements
Natural Products Insider https://www.naturalproductsinsider.com/probiotics/probiotic-health-benefits-natural-foods-and-supplements Steve DeMuri April 29, 2018Expanded Use of De Novo Pathway Offers Opportunity for Device Manufacturers
MedTech Intelligence Expanded Use of De Novo Pathway Offers Opportunity for Device Manufacturers Amy Scanlin April 23,...Insights into FDA’s Interpretation and Enforcement of Medical Foods
Independent Advisor for Labeling and Claims, Betty Campbell and Senior Director for Food Consulting Services, Allen Sayer have co-written an article on how FDA interprets and enforces regulations of Medical Foods in Food Safety Magazine. This narrow product category...Armstrong Discusses FSMA One Year Later in FDLI Update
Independent Advisor for Food Law and Regulation, Steve Armstrong discussed how FSMA implementation has impacted food safety systems during its first year of enforcement in the prestigious FDLI Update. Mr. Armstrong is the former Chief Law Counsel for Campbell Soup...Quality Assurance and Net Profits Discussed in EAS-Authored Article in Dairy Foods Magazine
EAS Independent Consultant, Rudy Westervelt, discussed fulfilling quality assurance potential and generating a net profit in a recent article published in Dairy Foods Magazine. Quality assurance could — and should — drive revenue by providing information that allows...EAS Releases Videos on FSMA and Product Development Services
EAS Independent Advisor for FSMA, Charles Breen, hosts a new informational video on EAS services in the complex area of the Food Safety Modernization Act, including FSVP, VQIP and more. FSMA is the largest overhaul to FDA’s food regulations in the last 70+ years and...Armstrong to Present at Food Law Innovative Conference
Independent Advisor for Food Law and Regulation, Steve Armstrong, will discuss Innovative Claims, as part of a USDA Review at the April 19-20, 2018 third annual CLE Food Law Conference in Denver. His co-presenter is Jeffrey Canavan, Deputy Director, Labeling &...EAS is Marquee Sponsor of TMA’s 103rd Annual Meeting and Conference
EAS President and COO, Dean Cirotta and Independent Consultant Karen Dixon will attend the Tobacco Manufacturers Association’s Annual Meeting and Conference taking place April 9-11, 2018 in Leesburg, VA. EAS is also a Marquee Sponsor of the event. The meeting will...Adverse Events, Serious or Not?
By Norma Skolnik This month’s Ask the Expert question on Serious Adverse Events Reporting is answered by Independent Consultant, Norma Skolnik. Norma has over 35 years of regulatory experience working with the pharmaceutical, OTC drug, and dietary supplement...March 2018 Drug and Device Corner
EAS would like to bring to your attention, the Statement from FDA Commissioner Scott Gottlieb, MD regarding drug compounding outsourcing facilities. The Drug Quality and Security Act (DQSA) was enacted by Congress in 2013 in response to a significant safety issue...Homeopathy – What Does the Future Hold?
By Susan Crane, Independent Advisor for OTC Labeling It’s been clear for several years that the FDA, as well as the Federal Trade Commission (FTC), are turning up the heat on homeopathic drug products. It culminated in December 2017 when the FDA published a...U.S. CODEX Office Relocated Within USDA, New Opportunities for the Food Industry
The Codex Alimentarius Commission (Codex) is a United Nations food standards setting body working under the auspices of the Food and Agriculture Organization and the World Health Organization (WHO). The official mission of Codex is to protect consumers’ health and...Buying a 21st Century Food Company
Natural Products Insider https://www.naturalproductsinsider.com/operations/buying-21st-century-food-company Steve Armstrong, J.D. March 29, 2018Co-Packers: ‘5 Golden Rules’ to Stay Competitive and Profitable
Natural Products Insider https://www.naturalproductsinsider.com/contract-manufacturing/co-packers-5-golden-rules-stay-competitive-and-profitable Heather Fairman March 27, 2018Interviewed for Article: “Clean” begins with “clear”- delivering on your labeling promise
MeatingPlace http://www.meatingplace.com/Member/Login?ReturnUrl=%2fIndustry%2fTechnicalArticles%2fDetails%2f78375 Steve Armstrong, J.D. March 26, 2018The Necessity of Food Safety Programs in Cannabis Food Processing
Cannabis Industry Journal The Necessity of Food Safety Programs in Cannabis Food Processing Gabe Miller March 21,...Supplier Quality Audits: A Critical Factor in Ensuring GMP Compliance
Cannabis Industry Journal Supplier Quality Audits: A Critical Factor in Ensuring GMP Compliance Amy Scanlin March 7,...Medical Foods – Insights Into FDA’s Interpretation and Enforcement
Food Safety Magazine https://www.foodsafetymagazine.com/enewsletter/medical-foods-insighte28099s-into-fdae28099s-interpretation-and-enforcement/ Elizabeth Campbell & Allen Sayler March 6, 2018Unraveling the Impact of FSMA On Acidified Food Regulations
Food Saety Tech https://foodsafetytech.com/feature_article/unraveling-impact-fsma-acidified-food-regulations/ Omar Oyarzabal, Ph.D. March 5, 2018Dietary Supplements vs. Foods — A FSMA Regulatory Challenge
In 1994 the Dietary Supplement Health and Education Act (DSHEA) created a new, legal class of food, called “dietary supplements,” which created a new subcategory of FDA-regulated foods. Many parts of the Food, Drug and Cosmetic Act (FD&C) still apply to dietary...Heather Fairman Answers Q&A in Natural Products Insider
EAS Independent Consultant Heather Fairman contributed to a Q&A in Natural Products Insider on Successfully Choosing and Maintaining a Contract Manufacturing Partnership. Heather was part of a panel of experts that discussed contract manufacturing aspects that...Food Quality and Safety Publishes EAS Authored Article on Audits and the Food Safety System
EAS Independent Consultant, Rob Carper, has written an article on how audits are an integral part of the food safety system for Food Quality and Safety Magazine. “Food safety plans must be monitored and verified throughout the year, making sure the entire written food...What’s New at FDA
The Center for Food Safety and Applied Nutrition has released the 2017 Food Code Book which includes science-based guidance for reducing known risks of foodborne illness. Originally designed for foods offered at the retail and food service locations, it has been...EAS Consultants Instructing FDLI Introductory Courses
EAS Consulting Group is honored to be a member of the Food Drug Law Institute and is pleased to be invited speakers to a number of upcoming events. Independent Consultant, Mark Nelson, will present an introductory session on Overview of U.S. Food Law and Regulation of...Does Our Company Offer Enough Training?
By Karen Dixon EAS is pleased to introduce a new column in our EAS-e-News called Ask the Expert. Each month our expert consultants and Senior Directors will answer one question sent in by readers (edited if applicable to remove identifying information). If you’d like...FSVP – Next Compliance Dates for Some Importers of Human and Animal Foods
By Charles Breen, Independent Advisor for FSMA The next FSMA/FSVP compliance date is March 19, 2018. FDA enforcement activities begin with: Importers of human food whose Small Business Foreign Supplier is required to comply with Preventive Controls for Human Foods...
Kathy Knutson, Ph.D.
Kathy Knutson, Ph.D. is a microbiologist and certified lead instructor for Preventive Controls for Qualified Individuals through the Food Safety Preventive Controls Alliance. She consults with companies in meeting FSMA requirements, including manufacturers in the...Have a Cup of Coffee and Pray – It’s the 20th Anniversary of the Seafood HACCP Rule
By Tim Hansen, Independent Consultant HACCP, of course, stands for Hazard Analysis Critical Control Point, is a highly effective preventive control system for foods and other commodities. “Have a cup of coffee and pray” was how one skeptical USDA union official...
Robert Burns, Ph.D.
Robert “Robbie” Burns, Ph.D. is an expert in infant formula. His position prior to consulting he was Vice President of Health and Nutrition Policy with the Grocery Manufacturers Association. Prior to GMA, Dr. Burns served as Global Nutrition and Scientific Affairs...Fulfill your quality assurance potential and generate net profit
Dairy Magazine https://www.dairyfoods.com/blogs/14-dairy-foods-blog/post/92755-fulfill-your-quality-assurance-potential-and-generate-net-profit Rudy Westervelt February 27, 2018The Ugly Duckweed: Will It Grow Into a Swan?
Natural Products Insider https://www.naturalproductsinsider.com/foods/protein-innovation Susan Moyers Feb 15, 2018Successfully Choosing and Maintaining a Contract Manufacturing Partnership
Natural Products INSIDER https://www.naturalproductsinsider.com/contract-manufacturing/successfully-choosing-and-maintaining-contract-manufacturing-partnership Heather Fairman February 13, 2018FDA Responds to GAO’s Report on Insufficient Agency Oversight of Recalls
By Charles Breen, Independent Advisor for FSMA The December 2017 Department of the Inspector General report on FDA’s food recall process included some particularly harsh criticisms of the agency including inadequate authority, oversight and follow-up on voluntary food...FDA’S Policy on Medical Foods
By Elizabeth Campbell, EAS Independent Advisor for Labeling and Claims “Medical food” is a commonly misunderstood term as it is used on FDA regulated products. Many manufacturers want to market their products as medical foods because medical foods are exempt from the...EAS President and COO to Speak at Keller and Heckman E-Vapor and Tobacco Law Symposium
Keller and Heckman’s Second Annual E-Vapor and Tobacco Law Symposium take place in Irvine, CA February 6-7, 2018. This comprehensive 2-day course will address regulatory issues relevant to e-vapor, e-liquid and tobacco product manufacturers, distributors and retailers...Armstrong to Moderate Session at FDLI Webinar on First Amendment Issues in Advertising and Product Packaging
Due to overwhelming response at the December FDLI Enforcement, Litigation, and Compliance Conference on the topic of First Amendment Issues in Advertising and Product Packaging, FDLI will be hosting a webinar on subject February 13, 2018, from 2:00-3:30 pm ET. EAS...Medical Device Quality Auditing
Medical Device manufacturers understand the tight FDA regulations and legal obligations surrounding devices. From initial safety and effectiveness study design through Pre-Market Applications (PMAs), Pre-Market Notifications (PMN), registration, Good Manufacturing Practices (GMPs) through reporting …

An Overview of Drug Master Files
DMFs are regulatory submissions to FDA for drug substances, drug products, and/or container closures allowing FDA to review information such as confidential details about facilities, processes, components, or articles used in the manufacturing, processing, packaging, and …
FDLI Publishes Article on The Value of FDA Pre-Submission Meetings & Enhancements under PDUFA VI
The prestigious FDLI Update November/December 2017 Student Corner included an article written by EAS Regulatory Intern and recent Georgetown University graduate, Rahul. The article, The Value of FDA Pre-Submission Meetings & Enhancements under PDUFA VI, discusses...Cirotta to speak at Keller & Heckman Forum on e-Vapor and Tobacco
EAS President and COO, Dean Cirotta will speak at the February 6-7, 2018 Second Annual E-Vapor and Tobacco Law Symposium in Irvine, CA. This comprehensive 2-day course will address regulatory issues relevant to e-vapor, e-liquid and tobacco product manufacturers,...Pharma’s Problems with Data Integrity
By Independent Consultant, Brian Nadel Data Integrity needs to be taken seriously in the pharmaceutical industry today and always! The FDA has issued many Warning Letters and Import Alerts to dosage form and Active Pharmaceutical Ingredient (API) manufacturers in the...Ask the Expert January 2018
By Allen Sayler EAS Webinar on FDA Draft Guidance: “Control of Listeria monocytogenes in Ready-To-Eat Foods: Guidance for Industry” October 31, 2017 EAS received an overwhelming response to our recent webinar on controlling Listeria monocytogenes in ready-to-eat...“The Food Safety Modernization Act, After One Year: Advancing and Building Food Safety Systems for the 21st Century”
https://www.fdli.org/2018/02/update-food-safety-modernization-act-one-year-advancing-building-food-safety-systems-21st-century/ FDLI Update Steve Armstrong, Independent Advisor for Food Law and Regulation...Produce Safety Requirements for Large Firms
By Charles Breen, Independent Advisor for FSMA The coming year will be a busy one for food firms as the compliance date for 14 components of the FSMA Final Rule come into effect. FDA has published a helpful tool, Key Dates for Compliance, which runs through 2024 and...
Stephen R. Cammarn, Ph.D.
Stephen Cammarn is an expert in pharmaceuticals and personal care products with a particular focus on quality assurance of manufacturing, research and development. Prior to consulting Dr. Cammarn built a career at The Procter & Gamble Company where he oversaw...December 2017 Drug and Device Corner
We would like to highlight one of the Draft Guidance issued by the FDA this month.**The U.S. Food and Drug Administration proposed a new, risk-based enforcement approach to drug products labeled as homeopathic. This proposed new approach would update the FDA’s...Why Audits Are Part of the Food Safety System
Unraveling The Impact of FSMA On Acidified Food Regulations
This webinar hosted by EAS Consulting Group will provide you with these answers. The webinar will be presented by EAS Independent Consultant Omar A. Oyarzabal, Ph.D., a microbiologist processing authority, and FDA Consumer Safety Officer Priya Rathnam with the Office of Compliance …
GMP and Other Requirements for Dietary Supplement Contract Manufacturers
Natural Products INSIDER https://www.naturalproductsinsider.com/manufacturing/gmp-and-other-requirements-dietary-supplement-contract-manufacturers Robert Fish December 14, 2017FDA Allows Co-Manufacturers More Time to Comply With Supply-Chain Program Requirements
In a nod to one of the more complex aspects of current food manufacturing and FSMA compliance, FDA recently published guidance that it will exercise enforcement discretion for two years over supply-chain requirements for domestic co-manufacturers of human and animal...Validating a Contract Laboratory – Infographic
Natural Products INSIDER https://www.naturalproductsinsider.com/labstesting/infographic-validating-contract-laboratory Tara Lin Couch, Ph.D. November 20, 2017
The Role of Regulatory Affairs in Product Development
Regulatory Affairs touches each and every product within a company, ensuring compliance with local regulations and internal policies and the creation of sound guidelines related to labeling, safety, and nutrition. Through the strategic links to trade associations, Government …
FDA Issues Guidance on Facilities Exempt from CGMP and Preventive Controls
One of the first steps for assessing a facility’s compliance status under the rules implementing the Food Safety Modernization Act is to consider whether the facility is required to comply or if it may qualify for an exemption, however narrow it may be. As a simple...What to Expect from SQF Modifications
FDA Proposes Four-Year Compliance Delay for Produce Rule’s Water Standard
FDA issued a proposed rule September 13, 2017, that would extend the compliance dates for agricultural water requirements in the Produce Safety Rule, giving the agency four years to reconsider the water standards to ensure that they are feasible. FDA Commissioner...U.S. Dairy Industry Challenge – How Many Food Safety Plans Are Enough?
By Allen Sayler, Senior Director for Food and Cosmetic Consulting Services The U.S. dairy processing industry is facing a significant challenge as it moves toward compliance with the FDA Food Safety Modernization Act (FSMA). This is best captured by a conversation I...
Getting Your Medical Device Into the US Market
The White Paper provides real-world and practical information directed to the manufacturer of Class II and Class III devices in navigating the Premarket Notification 510(k) clearance with insight into the various documentation required by FDA for submitting a 501(k)…
FDA Issues New Food Defense Guide, Food Safety Software
FDA released two useful tools last month to advise and assist companies in complying with new FSMA rules. The agency issued a Small Entity Compliance Guide(SECG) to help small food businesses comply with the FSMA final rule on Mitigation Strategies to Protect Food...Tips for Auditing a Contract Laboratory
By Tara Lin Couch, Ph.D., EAS Senior Director, Dietary Supplements and Tobacco Contract laboratories provide an extremely valuable and necessary service to the dietary supplement industry. The own-label distributor or manufacturer rarely has an in-house laboratory...FDA Funds States’ Efforts to Implement FSMA Produce Rule
FDA awarded almost $31 million in funding last month to help states implement FSMA produce safety programs — on top of the almost $22 million the agency awarded last fall for that purpose. The year-two funding underscores the importance of state partnerships as...Getting Your Medical Device Into the U.S. Market
By EAS Independent Consultant, Joe Ouellette If you are a manufacturer whose medical device is either a Class I, or a 510(k) exempt device Class II device, consider yourself and your company lucky that you do not have to try to “thread the needle” of the litigious and...FDA Draft Guidance on New Dietary Ingredients: Do you Need to File?
Tablets and Capsules July 1, 2017 https://tabletscapsules.com/wp-content/uploads/pdf/tc_20170701_0032.pdf Amy ScanlinChanges in What Constitutes ‘Dietary Fiber’ for Nutrition Facts Labeling May be in the Works
By Bruce Silverglade, EAS Product Development and Labeling Consultant One of the more controversial aspects of the FDA’s final rule on revising the Nutrition Facts Panel (NFP), 81 Fed. Reg. 33742(May 27, 2016), changes the definition of “Dietary Fiber” as it is...FDA Unveils Accredited Third-Party Certification Site
In one more step toward improved oversight of imported foods, FDA unveiled a new section of the FDA Industry Systems (FIS) websiteon June 21, 2017, to allow organizations – including foreign governments and agencies or private third-parties – to apply for recognition...Foreign Supplier Verification Program – What Does It Mean For Your Business
If you export food, food ingredients, food packaging, dietary supplements or food additives to the United States, your U.S. Importer will soon require that you provide documentation verifying that your company is compliant with the Food Safety Modernization Act (FSMA) regulations from the U.S. Food and Drug …
Process Labeling – Pathway to Transparency?
By Steve Armstrong, EAS Independent Advisor, Food Law & Regulation A thirty-year old federal court opinion that is well known to students of food and drug law holds that in the absence of a detailed, Congressionally mandated definition, courts and regulators...First FSVP Compliance Deadline Arrives
In a significant FSMA milestone, May 30, 2017 was the initial deadline for U.S. food importers to implement a foreign supplier verification program (FSVP). Beginning May 30, the U.S. Customs and Border Patrol (CBP) Automated Commercial Environment (ACE) system is...EAS Offers U.S. Agent Services for Foreign Companies
By Bryan Coleman, EAS Senior Director Pharmaceutical & Device Consulting Services, and Victoria Pankovich, EAS Regulatory Specialist The FDA’s regulatory requirement for foreign firms to have a U.S. Agent can be confusing, to say the least. There are very specific...Book Chapter: Food Products in the Fundamentals in US Regulatory Affairs, 10th Edition
FDA Combines Old and New Inspection Tools for Imported Foods
The Food and Drug Administration is using a combination of old and new tools to improve the safety of imports and a quick look at the numbers explains why. We’ve seen the familiar statistics: Almost 50 percent of fresh fruit, 20 percent of fresh vegetables, and 80...Safe Quality Food – Mitigation Strategies for Food Safety Fundamentals
The Facts About Supplement Facts
Wholefoods Magazine The Facts about Supplement Facts Susan Crane April 19,...The Food Processing Industry’s Newest Challenge: Safe Quality Food’s Edition 8.0
By Allen Sayler, EAS Senior Director for Food and Cosmetic Consulting Services The Safe Quality Food Institute (SQFI) is coming close to releasing its long-anticipated Edition 8.0. While many things could change during the final review and “sign-off” by the SQFI...Interview: Monitoring ingredient suppliers is imperative

Compliance With EU Food Regulation: What if Traceability and Food Recall are Not Enough?
EAS Consulting Group, LLC, leaders in FDA regulatory consulting is pleased to release a joint White Paper prepared by Italian business associate, Almater Food Technologists and Consultants srl (Italy) and LEXMA, who provide consulting services to food firms wish to …
Understanding the Drug-Cosmetic Conundrum When Marketing Your Products
By John Bailey, EAS Independent Advisor, Colors and Cosmetics and Catherine Bailey, Independent Consultant Products marketed as cosmetics can easily find themselves the target of FDA compliance action based on improper claims and statements. FDA is charged with...FDA Urges Industry Groups to Develop FSMA Guidance
By Charles Breen, EAS Independent Advisor, FSMA Consulting Services The Food and Drug Administration is encouraging industry groups and trade associations to develop their own guidance to help their members comply with new requirements under the Food Safety...Using Temporary Marketing Permits for Food Products
By EAS Independent Consultant Geraldine June FDA food standards of identity are regulations that establish the names and define the basic nature of certain foods. Food standards prescribe, among other things, what ingredients are required or optional and may describe...FDA’s Revised Draft Guidance on Listeria Controls
By Stephen Sundlof, D.V.M., Ph.D., EAS Independent Advisor, Animal and Human Food Safety FDA released updated draft guidance on preventive controls for Listeria monocytogenes in ready-to-eat foods last month, adopting the “seek and destroy” approach used by USDA’s...Recent Developments in EU Food Regulation
By Alfredo Gris, Daniele Pisanello and Massimo Scuccato As part of a new series in EAS-E-News on services provided through EAS partnerships, Italy-based food technology and consulting firm Almater shares the following insights on regulatory developments in the EU in...Third-Party Certification Will be Critical for Import Safety
By Stephen Sundlof, D.V.M., Ph.D., EAS Independent Advisor, Animal and Human Food Safety The Food and Drug Administration released an amended final rule last month establishing user fees so the agency can administer the FSMA program for accreditation of third-party...On FDA’s Latest FSMA Guidance
The Food and Drug Administration recently issued three new guidance documents to help simplify compliance with Food Safety Modernization Act (FSMA) regulations and programs. On November 7, the agency released new draft guidance on registration of food facilities. The...Drug Master File Submissions – An Overview
By Naitry Shah, EAS Intern (This article summarizes a White Paper on DMF submissions prepared by EAS Independent Consultant Albert Yehaskel.) Drug Master Files (DMFs) are detailed submissions to the Food and Drug Administration (FDA) that may be used to provide...FDA Invites Comment on Two Draft CGMP Guidances
FDA is inviting public comments by November 23 on two draft guidances — on current good manufacturing practices (CGMP) requirements for food for animals and on CGMPs for human food by-products for use as animal food. The two guidances are essential reading if...What Now for FSMA Compliance?
The sky did not fall on September 19, 2016, the compliance date for larger human food facilities to meet FSMA preventive controls and Current Good Manufacturing Practice (CGMP) requirements, and for larger animal food facilities to comply with CGMPs. In a September 19...
Analysis of Final FDA Regulations Establishing New Reference Amounts Customarily Consumed (RACCs), New Requirements for Single Serving Containers, and Dual Column Labeling
Disclosures on the Nutrition Facts label are based on serving sizes derived from Reference Amounts Customarily Consumed (“RACCs”) that are determined by FDA. FDA has changed its RACCs for dozens of food categories, affecting the number of nutrients for customary …
Why FDA Views Registration as a FSMA Priority
The Food Safety Modernization Act’s provisions on food facility registration are especially significant from FDA’s perspective because they will enable the agency to know much more about the businesses whose products are covered by the landmark law. On July 14, 2016,...FDA’s Design Control Requirements for Medical Devices
By Kaiser Aziz, PhD., EAS Senior Consultant FDA reviewers and field investigators evaluate design control requirements and processes for medical devices and make recommendations based on whether the manufacturer has the required checks and balances in place. Design...
FDA’s Design Control Requirements for Medical Devices
FDA reviews, evaluates, verifies and validates the implementation of the design control requirements described in the device premarket applications (510k/ PMA). Design control requirements play a key role from the device design prototype, the manufacturing process to …

Analysis Of Final FDA Regulations Amending The Nutrition Facts Label – Focus On Added Sugars
This White Paper provides an analysis of FDA’s final regulation amending the Nutrition Facts label, focusing on FDA’s new requirement for added sugars content disclosure and disclosing a %DV for added sugars. The impacts of other provisions of the final rule related to the …
How to Successfully Respond to FDA 483s and Warning Letters
EAS Senior Advisor for Quality and Compliance, Mr. Robert Fish, will present a webinar on How to Respond to FDA 483s and Warning Letters. He will discuss the correct and incorrect way to prepare these responses with emphasis on the FDA’s expectations. He will also explain why companies may receive an FDA 483 and how to respond in a way to prevent the issuance of a Warning Letter.”
FDA Unveils FSMA Final Rule on Intentional Adulteration
FDA has now released its seventh FSMA rule – on intentional adulteration. Published May 27 in the Federal Register, the rule requires registered food facilities to develop a written food defense plan to address intentional adulteration. In an approach similar to...FSMA and Dietary Supplements
By Charles Breen, EAS Senior Advisor for FSMA Consulting Services FSMA’s preventive controls rule, 21 CFR 117.5(e), exempts finished dietary supplements (DS) from the requirements for preventive controls and a supply-chain program if they are in compliance with 21 CFR...Are You ‘Qualified’ under the Preventative Controls for Human Food Rule?
Who is “qualified” under FDA’s final rule on preventive controls for human food? Why would someone want to be “qualified?” What is the role of someone who is “qualified?” How much of a role does a “qualified” person play to ensure a safe and wholesome food supply? The...FSMA Final Rule on Sanitary Transportation of Food Includes Major Revisions
FDA released the FSMA Final Rule on Sanitary Transportation of Human and Animal Food on April 6, 2016. The final rule, which is part of the agency’s implementation of the 2005 Sanitary Food Transportation Act, contains some significant revisions of the proposed...What Makes a Good Sampling Plan?
By EAS Senior Consultant William R. Fairweather, PhD. You have a question, so you design a study to explore it. You determine that A is better than B and that the average difference is 3 units. It should be obvious that this is not sufficient information, but why not?...Implementing FSMA for Imports is FDA’s Biggest Challenge
FDA hosted an excellent day-long public meeting on March 21, 2016 to review the import-related elements of the new FSMA regulations. In opening remarks, FDA Deputy Commissioner for Foods and Veterinary Medicine Michael Taylor noted that implementing import provisions...The Purpose of a DEA Regulatory Investigation
By EAS Senior Consultant Karen Famiglietti Everyone has read recent newspaper and internet stories about the rise in heroin abuse and its relationship with pharmaceutical opiates that were originally prescribed for legitimate medical reasons. Unfortunately, this...FSMA and Third-Party Auditing
A key piece of the regulatory jigsaw puzzle as FDA moves ahead with implementing the Food Safety Modernization Act (FSMA) is the voluntary, user-fee based program for the accreditation of third-party certification bodies to conduct food safety audits of foreign food...What Are Medical Foods (and What They are Not)
By Jeanne Hoskin, Ph.D., EAS Senior Consultant Prior to 1972 medical foods were regulated as prescription drugs under section 201(g)(1)(B) of the FD&C Act, requiring manufacturers to conduct drug trials and submit Investigational New Drug license applications and...Produce Final Rule Goes into Effect
The Food Safety Modernization Act (FSMA) extended FDA’s authority to include produce all the way to the farm level, which put the agency in unfamiliar regulatory territory. To avoid burdening covered facilities with unreasonable compliance demands, the agency...FDA Responds to Comments in FSVP Final Rule
FDA released three significant FSMA final rules last month, on foreign supplier verification, produce and accreditation of third-party auditors – all formally published in the Federal Register on November 27. Each of these three significant rules deserve separate...Getting to Grips with the electronic Common Technical Document (eCTD) Process
By Albert Yehaskel Completing a successful electronic Common Technical Document (eCTD) submission can be daunting, even with the help of guidance documents from the International Conference on Harmonization (ICH) – which developed the eCTD – or from...FSMA Implementation Enters a New Phase
The Food and Drug Administration hosted a public meeting in Chicago on October 20, 2015 to discuss the recently released final rules on preventive controls for human and animal food, along with plans for the next phase of FSMA implementation. This was a substantive...New Drug Development in the 21st Century
By Nancy Chew Most people who work in the pharmaceutical industry know that drug development comprises of pharmaceutical development, animal pharmacology and toxicology studies, and clinical research; many also know that there are quality standards applied to...Managing FDA Inspections
With FDA inspections becoming more frequent, particularly in the dietary supplement world, it is imperative that companies familiarize themselves with how to host and manage such an inspection. We say “manage an FDA inspection” because the more knowledge a company has the better able it will be to make the inspection process move smoothly in the direction that you help set. To provide you with that knowledge, this webinar will cover: