Thinking about an FDA gap assessment this year? Contact EAS now to schedule your 2023 audit. Schedules toward the end of the year tend to book up suddenly, now is the time to lock in your audit, whether it be onsite or virtual. We would be happy to prepare an audit proposal so you can review and schedule prior to Dec 2023.
The FDA is stepping up enforcement of their Electronic Registration and Listing Compliance Program which is in place to ensure accurate drug establishment registration, and NDC listing information. EAS has seen quite a few notifications from FDA to clients advising the need to correct invalid NDC listings. If an update has been overlooked, it is a simple process to update and bring your listing into compliance. Failing to respond to the agency’s request will lead to NDC listings being removed from the FDA’s public database, and DailyMed listings to show as inactivated. This is a good reminder that company should be reviewing their NDC listings every June and December at a minimum. If you have any questions on your listing status, EAS is here to assist as your regulatory partner.
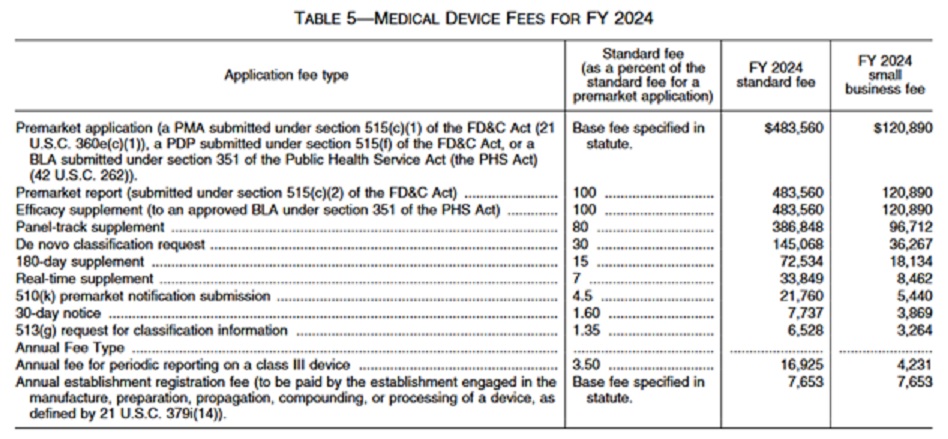
The FDA has announced Medical Device User Fee Rates for Fiscal Year 2024 in the Federal Register Vol 88, No 144.
Highlighted Guidance Documents
This guidance describes a program at FDA’s Center for Drug Evaluation and Research to make public a comprehensive listing of recognized voluntary consensus standards related to pharmaceutical quality. FDA’s participation in the development and use of technical voluntary consensus standards has been integral to the execution of FDA’s mission.
This guidance provides recommendations for presenting quantitative efficacy and risk information in direct-to-consumer (DTC) promotional labeling and advertisements for prescription human drug and biological products and prescription animal drugs and in DTC promotional labeling for over-the-counter animal drugs (collectively, promotional communications). For the purposes of this guidance, quantitative efficacy and risk information refers to information that numerically addresses the likelihood or magnitude of a drug’s efficacy or risks.
Prohibition on Wholesaling Under Section 503B of the Federal Food, Drug, and Cosmetic Act
This guidance describes FDA’s interpretation of, and policies concerning, the prohibition on wholesaling in section 503B of the FD&C Act. This guidance also describes examples of how FDA intends to apply section 503B’s wholesaling provision.
Qualification of Medical Device Development Tools
This guidance describes a voluntary program for the qualification of medical device development tools (MDDTs) for use in the evaluation of devices regulated by CDRH. Specifically, this guidance describes the framework for voluntary proposal and qualification of an MDDT, including definitions of applicable terms, criteria for evaluating an MDDT for a specific context of use, considerations for qualification, and the contents of a qualification package.
This draft guidance provides recommendations for the design of pivotal clinical studies for devices intended to treat OUD and used to support marketing submissions. These recommendations are applicable to the design and development of clinical studies to provide a reasonable assurance of safety and effectiveness for a device intended to treat OUD.
All Guidance Documents can be searched on the FDA’s website.
Federal Register
FR Vol 88, No 83 Draft Pharmaceutical Quality/Chemistry Manufacturing and Controls Data Elements and Terminologies; Establishment of a Public Docket; Request for Comments
FR Vol 88, No 123 Presenting Quantitative Efficacy and Risk Information in Direct-to-Consumer Promotional Labeling and Advertisements; Guidance for Industry, Availability
Meetings
OTC Monograph Reform: OMOR Format and Content & Electronic Submissions
Date: August 22, 2023
Time: 1:00 PM – 2:00 PM ET
Dates: September 12 – 13, 2023
Day1: Tue, Sep 12 7:00 AM – 10:00 AM ET
Day2: Wed, Sep 13 7:00 AM – 10:00 AM ET
Advancing Generic Drug Development: Translating Science to Approval 2023
Dates: September 13 – 14, 2023
Day1: Wed, Sep 13 9:00 AM – 5:00 PM ET
Day2: Thu, Sep 14 9:00 AM – 5:00 PM ET
Date: September 14, 2023
Time: 1:00 PM – 2:00 PM ET
Date: Tue, Nov 7, 2023
Time: 9:00 AM – 12:00 PM ET (this is meeting 2 of a 2 meeting series)
FDA Websites of Interest
Manual Of Policies And Procedures, Manual Of Policies And Procedures Center For Drug Evaluation And Research Mapp 5014.1 Rev. 1, Office Of Pharmaceutical Quality, Understanding CDER’s Risk-Based Site Selection Model
Office of Global Policy and Strategy Statement – 27 July 2023, FDA Mutual Recognition Agreement with Swissmedic Enters Into Force
CDRH Announces New Standards Recognition to Support Innovation in Medical Device Sterilization
FDA Takes Steps to Facilitate Innovation for Devices Intended to Treat Opioid Use Disorder
Posted in Drug and Device Corner, Drugs, Medical Devices.
