Prepared by Bruce Silverglade, Independent Consultant, EAS Consulting Group, LLC
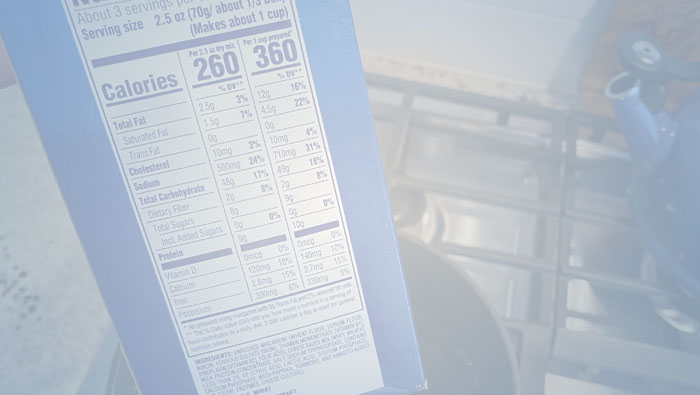
Disclosures on the Nutrition Facts label are based on serving sizes derived from Reference Amounts Customarily Consumed (“RACCs”) that are determined by FDA. FDA has changed its RACCs for dozens of food categories, affecting the number of nutrients for customary servings of foods that companies must list on the Nutrition Facts label. FDA has also changed its regulations on single-serving packages, again affecting the number of nutrients that will need be disclosed on the label. Thus, FDA’s new regulations have a direct impact on the amount of fat, sodium, and sugars that companies must disclose on the Nutrition Facts Label. Some foods may appear higher in these nutrients than before the new regulations. This paper analyzes the changes and discusses implications for food companies.
Posted in Foods, White Paper and tagged Bruce Silverglade.