EAS Blog
EAS Independent Consultants and staff provide in-depth regulatory expertise and authoritative reporting on a variety of compliance requirements for industries that EAS serves. Our experts break down complicated topics into easily understandable summaries and analysis. Subscribe to our newsletter and receive these monthly updates directly in your in-box.
Dairy Processing 101
A Virtual Training Presented by EAS Consulting GroupPresented by EAS Independent Consultants, Allen Sayler, Gabe Miller, Dave Blomquist, and Rhaisa A. Crespo, Ph.D.February 15, 17, 22 & 24, 2022From 12:00pm-3pm eastern each dayThe FDA’s Food Safety Modernization ACT (FSMA) and Safe Quality...
EAS Welcomes Maged Sharaf, Ph.D. as Senior Director, Labeling, Cannabis and Claims Consulting Services
Alexandria, VA: EAS Consulting Group is pleased to welcome Maged Sharaf, Ph.D. to the management team. As Senior Director, Labeling, Cannabis and Claims Consulting Services, he will be responsible for managing and providing label review and claims substantiation consulting services for...
Titanium Dioxide – What the EU Ban Means For You
By April Kates, EAS Independent Consultant What is Titanium Dioxide? Titanium dioxide is a substance that has regulatory status in the US for many uses, including as a food additive. In 1966, It was approved to be used in foods at up to 1 percent by weight as a color additive. As a coloring agent,...
Drug and Device Corner November 2021
Final reminder that we are entering the last month of the registration renewal period for medical device facilities and drug establishments, as well as drug listing certifications. The FDA has provided an Electronic Drug Registration and Listing Instructions website for support. Clients wishing to...
Amazon Offers New Documentation Flexibility for Dietary Supplement Selling Partners
Amazon recently announced flexibility for selling partners to demonstrate of Good Manufacturing Practices (GMPs) for dietary supplements. The new policy requires submission of three demonstrations of quality, two of which are mandatory. The third requirement provides flexibility, with two options...
Dietary Supplement Good Manufacturing Practices (GMP) for Laboratories
Ensuring Regulatory CompliancePresented by Charlotte Peyton, Independent ConsultantMarch 1 & 3, 2022, from 12-4:30 pm eastern each dayEAS is offering an intensive virtual seminar covering FDA’s current Good Manufacturing Practice (GMP) requirements for Research and Development and Quality...
EAS Expert Interviewed on The Safety of Imported Ingredients in Food Engineering Magazine
EAS independent consultant, Susan Moyers, Ph.D. was interviewed on regulations and safety of imported food ingredients in Food Engineering Magazine. Learn about FDA and USDA oversight of imported foods as well as particular challenges surrounding standards of identity.
From Great Nana’s Recipe to Store Shelves: Things to Consider When Scaling Up
Part 1: LabelingWhether it is that recipe that has been handed down from generations, or the one product that is the star of your restaurant or catering business, or perhaps, even something you have spent years perfecting, taking the leap to commercial scale-up can be an exciting but daunting...
Regulatory Considerations for Novel Sports Nutrition Products and Ingredients
By EAS Independent Consultant, David Cockram, PhD Nearly every athlete wants something extra that will provide them with an edge over the competition. Optimizing nutrition is certainly a huge part of improving athletic performance. A well-balanced diet, with appropriate levels of carbohydrates,...
Drug and Device Corner October 2021
REMINDER that we are in the renewal period for all drug establishment, and medical device facility registrations, as well as the drug listing certification period. The agency announced this month their intention to withdraw the Temporary Guidances for Alcohol-Based Hand Sanitizers. With a thank...
Top Ten Fatal Flaws in your Food Safety Plan
By Bryan Armentrout, EAS Independent Consultant. “Is your food safety plan ready for a U.S. Food and Drug Administration (FDA) inspection?” Did you know that the average cost of a recall is now over $30 million? That is actual cost; it doesn’t include losses to brand value, lawsuits, and lost...
Are You Producing Alcohol-Based Hand Sanitizers?
FDA has announced the withdrawal of temporary guidances for alcohol-based hand sanitizers manufactured by non-drug manufacturers during the COVID-19 public health emergency. Effective December 31, 2021, companies manufacturing alcohol-based hand sanitizers under these temporary policies must cease...
When is a Cosmetic Also a Drug?
Did you know that products designed to clean and beautify that ALSO affect the structure or function of the human body must bear special labeling? Per FDA’s 21 USC 359, these combination cosmetic – OTC Drug products must comply with OTC drug monographs and bear “Drug Facts” labeling for drug...
Sunscreen Quality, Safety and Efficacy
New Initiatives by FDA FDA has proposed revisions and updates to OTC-drug sunscreen requirements related to maximum sun protection factor (SPF) values, active ingredients, broad-spectrum requirements, and product labeling, among other provisions. This effort aims to ensure adequate ultraviolet A...
FDA Finalizes Two Foundational PMTA Rules
U.S. Food and Drug Administration has issued two final rules for the premarket review of new tobacco products, providing additional information on the requirements for the content, format and review of Premarket Tobacco Product Applications (PMTAs) and Substantial Equivalence (SE) Reports – two of...
Dietary Supplement Virtual GMP Refresher
Presented by EAS Independent Consultant Tamika CatheyJanuary 18-19, 2022 from 11am-3pm eastern The Good Manufacturing Practices (GMP) dictated in FDA’s 21 CFR 111 require that “Each person engaged in manufacturing, packaging, labeling, or holding, or in performing any quality control operations,...
Why You Should Outsource Your Food Safety Testing
As a member of the Certified Group and Food Safety Net Services, EAS clients have access to world-renowned testing laboratories that meet your organization’s sophisticated needs. In this column, you’ll hear about their capabilities, environmental challenges and more. We hope you’ll enjoy a look...
Social Media Considerations for FDA Regulated Products
By Susan Crane, Independent Advisor, OTC Drugs and LabelingHistorically, it has been straightforward for the FDA to review and monitor labeling and advertising for products under their jurisdiction, particularly drugs and medical devices. However, the widespread use of the internet and social...
Tim Lombardo Discusses Food Safety for CBD Infused Edibles in NCIA Podcast
Senior Director for Food Consulting Services, Tim Lombardo, recently joined NCIA for a podcast covering food safety of CBD-infused edibles.
USDA FSIS Revised Guidelines for Labeling Kit Food Products
Those producing nonretail-exempt, multicomponent food kits (such as stir fry and pizza) have a newly revised resource from FSIS: An eight-page booklet with criteria that helps to determine whether the kit product needs to be prepared under FSIS inspection and if so, how to label a kit product that...
Drug and Device Corner September 2021
As part of the 2020 CARES Act, section 506J has been added to the FD&C Act. This section requires manufacturers of certain medical devices to report any interruption or permanent discontinuance in manufacturing to the CDRH. The list of relevant devices can be found on this FDA website. A...
Food Safety – Of the Package By the Package and For the Package
Presented by EAS Independent Consultant, Thomas Dunn. Did you know the safety of your product begins and ends with packaging? Packaging is considered the “forgotten food ingredient” and has a myriad of US regulatory requirements including food contact substances, minimizing or preventing food...
Drug OTC GMPs and Labeling
A Five-Part Virtual Seminar SeriesPresented by Lisa El-Shall, Victoria Pankovich, Jeb HunterNew dates to be announcedIf you manufacture products in the OTC-drug space, including homeopathic, the challenges of FDA regulatory requirements become confusing. With a mixture of regulations spanning...
Food Labeling Modernization Act of 2021 Introduced
‘The Food Labeling Modernization Act’ (FLMA) was introduced this month in both houses of Congress by Representative Frank Pallone (D-NJ) and Senator Richard Blumenthal (D-CT), along with Representative Rosa DeLauro (D-CT) and Senators Ed Markey (D-MA) and Senator Sheldon Whitehouse (D-RI). The...
What’s Hot in the Regulatory World
By Richard D’Alosio, EAS Independent Consultant I have always described working in regulatory as an iceberg, with the obvious black and white answers to inquiries akin to the visible iceberg floating in the water. However, underneath the water is the unknown or unregulated areas, and that is where...
Managing Pet Food Ingredients
EAS Senior Director for Food Consulting is featured in an article on pet food ingredients. From safety, to testing to CoAs, the same safety standards apply as with human foods. Read more in Food Engineering Magazine.
Did you know? Color Additives Must Have FDA Approval and (Sometimes) Batch Certification
Color additives for most FDA-regulated products (tobacco and some medical device products are exceptions) must be approved by FDA and listed in the Code of Federal Regulations. If a color additive isn’t listed, its approval must be petitioned and approved by FDA prior to its use in products...
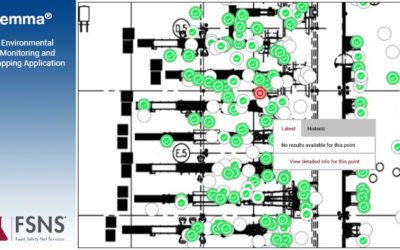
Environmental Monitoring and Mapping Application (emma®)
By Food Safety Net Services Environmental monitoring programs (EMPs) are critical in food processing environments to ensure the prevention and detection of pathogens. Many of these programs have evolved to mitigate risk of contamination and can serve as an early warning sign of risk to food...
Do you Make Good, Clean and Healthy Claims on Your Product Labels?
You may be misrepresenting your products by making claims such as good, clean and healthy. FDA has clearly defined definitions for some claims and deciding which to make to market your products can be tricky. Read the EAS authored article in Snack Food and Wholesale Bakery and learn how you can...
Drug and Device Corner August 2021
FDA has announced most FY2022 user fee rates this month, conspicuously missing are the OMUFA 2022 user fee rates. We will continue to monitor Federal Register notices and share information as soon as it becomes available. Federal Register Vol. 86, No. 142 announcing FDA 2022 GDUFA user fee rates...