Please see below information regarding FY2025 Medical Device User Fees which go into effect 1 October 2024. Keep in mind, the FY2025 facility user fee must be paid prior to processing your annual renewal during the renewal period Oct 1 – Dec 31.
FY2025 Medical Device facility registration user fees can be found on the FDA’s Medical Device User Fee Amendments (MDUFA) website. There are NO waivers or reductions of facility user fees for small establishments, businesses, or groups in FY2025. The $9,280 annual establishment registration fee must be paid PRIOR to submitting your annual renewal.
For businesses that may qualify as a ‘Small Business’ for CDRH’s Small Business Program, keep in mind an application must be submitted annually to continue to participate in the program. The Reduced Medical Device User Fees: Small Business Determination (SBD) Program website has full details and information on the process.
Please see FY2025 Medical Device User fees from the MDUFA website below:
User Fees for FY 2025
Annual Establishment Registration Fee: $9,280
All establishments must pay the establishment registration fee. There are no waivers or reductions for small establishments, businesses, or groups in FY 2025.
Other fees for Fiscal Year 2025 (October 1, 2024, through September 30, 2025) are:
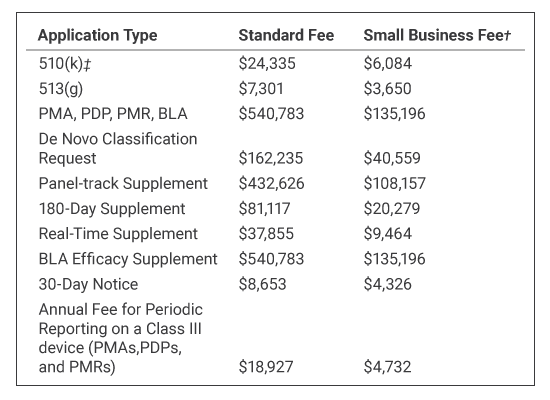
† Small Business Fee: For businesses certified by the Center for Devices and Radiological Health (CDRH) as a small business. For more information, see the section Small Businesses below.
‡ 510(k) Fees: All types of 510(k)s (Traditional, Abbreviated, and Special) are subject to the user fee. However, there is no user fee for 510(k)s submitted to the FDA on behalf of an FDA-accredited third-party reviewer.
Medical device user fee types can be found on the FDA’s webpage.
Posted in FDA and USDA Regulatory Update, Medical Devices.
