Please see below information regarding FY2025 Drug Program User Fees which go into effect 1 October 2024.
The FDA has announced FY2025 user fees for GDUFA, OMUFA (Order Requests only), PDUFA, BsUFA, and animal user fee programs. Please see the Federal Register notices for details on each.
FR Vol. 89, No. 147 Generic Drug User Fee Rates for Fiscal Year 2025
FR Vol 89, No. 147 Over-the-Counter Monograph Drug User Fee Program-OTC Monograph Order Request Fee Rates for Fiscal Year 2025
FR Vol 89, No. 147 Prescription Drug User Fee Rates for Fiscal Year 2025
FR Vol. 89, No. 147 Biosimilar User Fee Rates for Fiscal Year 2025
FR Vol. 89, No. 147 Animal Drug User Fee Rates and Payment Procedures for Fiscal Year 2025
Program specific websites with links below.
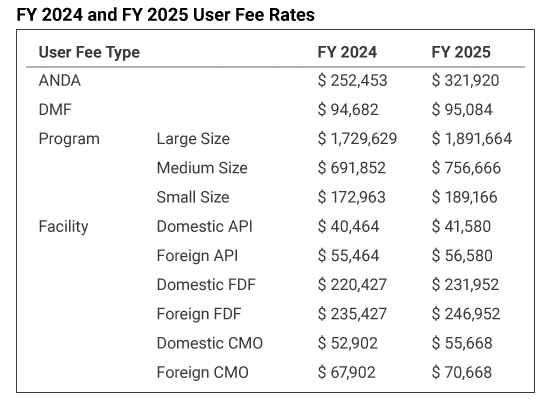
Generic Drug User Fee Amendments
FDA has updated the address for user fees delivered by courier, such as Federal Express or UPS. Beginning October 6th, to prevent any disruption of overnight packages, please send any payment arriving via courier to:
U.S. Bank
Attention: Government Lockbox 979108
3180 Rider Trail South
Earth City, MO 63045
If you have any questions concerning courier delivery, contact U.S. Bank at 800-495-4981.
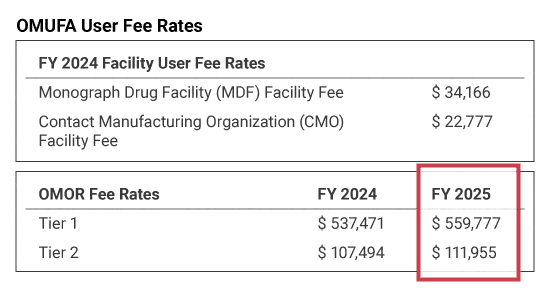
The OMUFA FY2025 Order Request user fees have been announced, facility user fees have not yet been announced. Facility user fees are not available until the FY2025 drug establishment renewal period ends. Typically, the agency announces OMUFA facility user fees in March. EAS will keep you informed of this announcement.
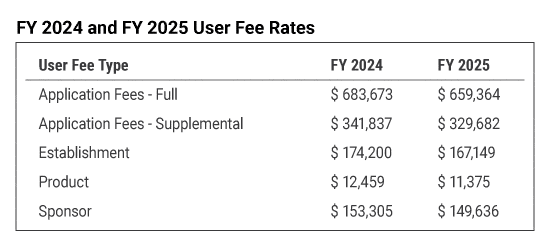
Prescription Drug User Fee Amendments
The FY 2025 PDUFA program fee invoices were emailed on Thursday, August 15, 2024. Full payment of the invoice is due on October 1, 2024. If you did not receive your invoice by August 19, 2024, please contact PDUFA User Fee staff at CDERCollections@fda.hhs.gov.

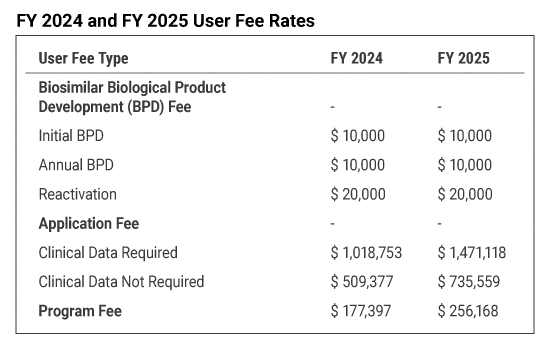
Biosimilar User Fee Amendments
The FY 2025 BsUFA annual invoices for annual BPD and/or program fees were emailed to sponsors in the BPD program and applicants with program-fee eligible biosimilar products on August 23, 2024. If you are expecting an invoice and do not receive it, please contact the BsUFA User Fee staff at CDERCollections@fda.hhs.gov. Invoices are due by October 1, 2024.
Animal Drug User Fee Act (ADUFA)
New address for mailing annual waiver requests:
Food & Drug Administration
Center for Veterinary Medicine
MPN 2, E150
Attention: ADUFA Waiver Officer
12225 Wilkins Avenue
Rockville, MD 20852
Posted in Drugs, FDA and USDA Regulatory Update.
